Answers section
Questions 1.4(a) 
- What would happen between electrons if they are very close to one another?
- What would happen between an electron and the positively charged nucleus?
-
- Describe the path taken by the ball as it moves.
- What kind of motion is this? (Hint: motion that causes seasons on the Earth).
- What happens when the swirling is stopped?
- What causes the behavior in 3(c)?
- What does the spring or elastic represent in this demonstration?
- When an object stops revolving or moving in a circle, it falls to the centre of the circle. True or False?
- During motion of electrons around the nucleus, they do not move to the nucleus; yet they are attracted there. What is keeping electrons away from the nucleus?
Observe the demonstration alongside Figure 1.4.1, to see why electrons do not fall to the nucleus.
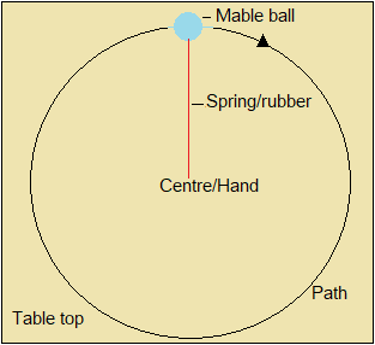
Figure 1.4.1: Marble ball whirled on a spring or elastic
Answers to Questions 1.4(a) 
- They would repel and move away from one another.
- The electron and nucleus would attract each other, and the electron would move to the nucleus.
- The path is circular, with the hand as its centre.
- Revolution (or orbital motion).
- The marble ball moves to the centre, where the hand is.
- The stretched elastic or spring pulls the marble ball to the centre.
- The stretched spring represents attraction between the electron and nucleus.
- True.
- Motion around the nucleus
