Answers section
Questions 4.4 
- Complete the equations numbered (b), (c), (e), (f), (h), (i) and (k).
- Name the salts produced in (a) to (f) and (h) to (i).
- From the equations, identify four methods to prepare salts.
NB:Use the following guide to indicate the correct state symbols.
- PbSO4, BaSO4, AgCl, and all carbonates except of Na+, K+, NH4+ are insoluble.
- All oxides and hydroxides of metals, except of Na+, K+, and NH4+ are insoluble.
- State symbol is (aq) for soluble and (s) for insoluble substances.
Answers to Questions 4.4 
-
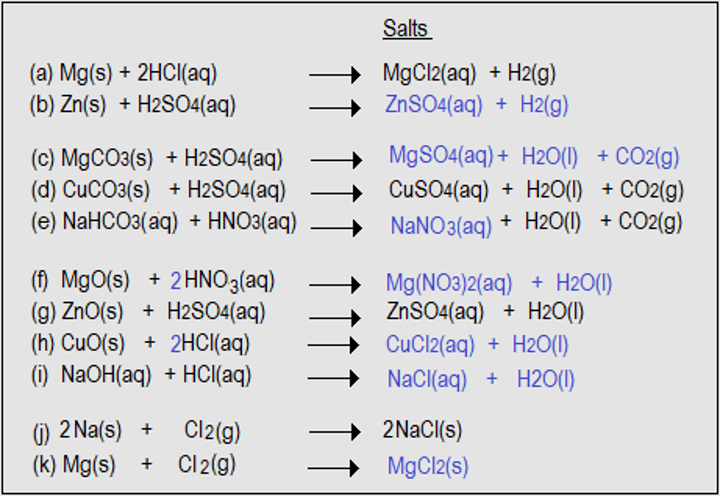
- Magnesium chloride
- Zinc sulphate
- Magnesium sulphate
- Copper (II) sulphate
- Sodium nitrate
- Magnesium nitrate
- Copper (II) chloride
- Sodium chloride
- Reacting a metal with an acid (dilute)
Reacting a metal carbonate or hydrogencarbonate with an acid
Reacting a metal oxide or hydroxide with an acid.
Reacting a metal (an element) with an element (Direct Combination of elements)
