Answers section
Questions 4.5.3 
- Complete Table 4.5.3 to indicate the observations and products formed.
- Write chemical equations for the reactions.
- What can you conclude about effect of heat on nitrates of heavy metals?
Answers to Questions 4.5.3 
-
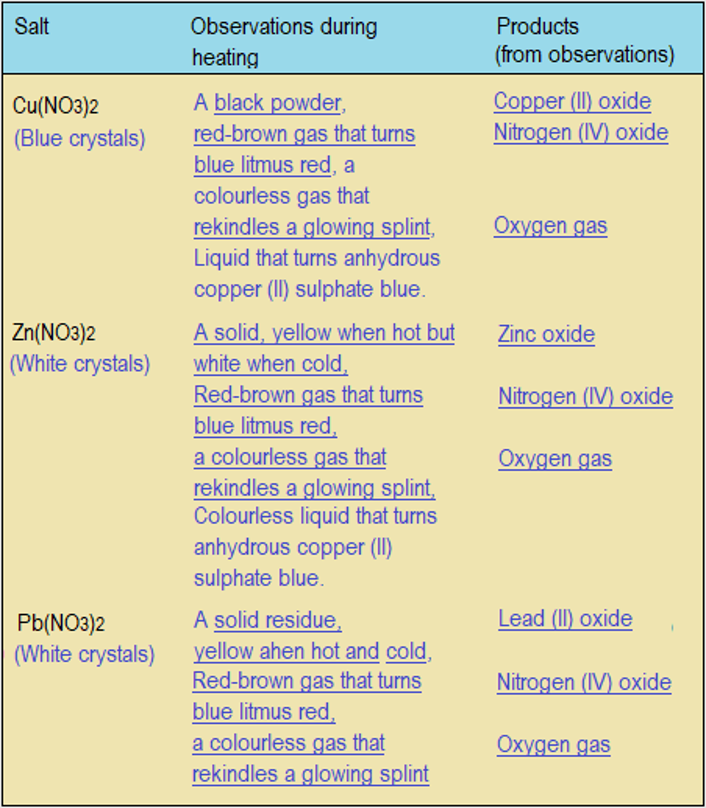
- Cu(NO3)2(s) ⟶ CuO(s) + 2NO2(g) + O2(g)
Zn(NO3)2(s) ⟶ ZnO(s) + 2NO2(g) + O2(g)
Pb(NO3)2(s) ⟶ PbO(s) + 2NO2(g) + O2(g) - Nitrates of heavy metals decompose on heating to form metal oxide, nitrogen (IV) oxide, and oxygen gas.
