Answers section
Questions 2.4(b)
- A compound X, consists of 3.5 g of nitrogen and 8 g of oxygen as the only components. Determine the empirical formula of X (N = 14; O = 16).
- If the molar mass of X (in question 1) is 92 g, find its molecular formula.
- In a compound, 4 g of hydrogen is found to be combined with 32 g of oxygen. Determine the empirical formula of the compound (H = 1; O = 16).
- The empirical formula of a compound is ZQ2. The masses of Z and Q in a sample of the compound are 2.5 g and 17.75 g respectively.
Determine the molar mass of (a) Z and (b) ZQ2
(Q = 35.5).
Answers to Questions 2.4(b) 
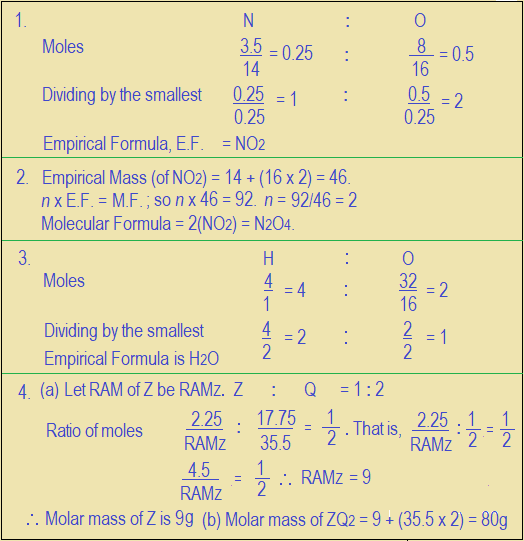
Plate 2.4b Answers to Questions 2.4(b)
