Answers section
Questions 3.1.7(b)
- State the observable change when methane is mixed with chlorine gas in diffused sunlight (in the room)?
- Suggest the role of sunlight in this reaction.
- During the reaction, each chlorine molecule splits into single atoms which are unstable (like in many other reactions). Then one
chlorine atom knocks off and substitutes (replaces) one hydrogen atom from methane, in a process called substitution reaction.
- What do you expect to happen to the other chlorine atom and the hydrogen atom substituted? Explain.
- Write the formulae of the two products at this stage.
- Write the equation for the reaction at this stage. Remember to write ultraviolet light over the arrow in the equation.
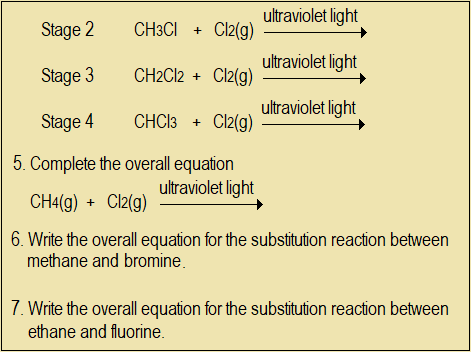
- Substitution reaction continues in stages till all the four hydrogen atoms in methane are replaced by chlorine atoms. Complete the following equations for the substitution reaction.
Answers to Questions 3.1.7(b) 
- Colour changes from pale green to colourless.
- Ultraviolet light is the energy required to split chlorine molecules into atoms.
- (a) Chorine atom combines with the displaced hydrogen atom to form hydrogen chloride gas. Both atoms are unstable.
(b) CH3Cl and HCl
(c) CH4(g) + Cl2(g) CH3Cl(g) + HCl(g)
CH3Cl(g) + HCl(g) - (a) CH3Cl(g) + Cl2(g)
 CH2Cl2(g) + HCl(g)
CH2Cl2(g) + HCl(g)
(b) CH2Cl2(g) + Cl2(g) CHCl3(l) + HCl(g)
CHCl3(l) + HCl(g)
(c) CHCl3(l) + Cl2(g) CCl4(l) + HCl(g)
CCl4(l) + HCl(g) - CH4(g) + 4Cl2(g)
 CCl4(g) + 4HCl(g)
CCl4(g) + 4HCl(g) - CH4(g) + 4Br2(g)
 CBr4(g) + 4HCl(g)
CBr4(g) + 4HCl(g) - CH3CH3(g) + 6F2(g)
 CF3CF3(g) + 4HCl(g)
CF3CF3(g) + 4HCl(g)
