Answers section
Questions 4.7
- Prepare a one-page summary for the topic, nitrogen and its compounds. Refer to "Answers to Questions" sections where you find it necessary.
- Explain the chemical tests and results to confirm presence of:
- Ammonia gas (NH3)
- Nitrogen (I) oxide (N2O)
- Nitrogen (II) oxide (NO)
- Nitrates (or nitrate ions, NO3-)
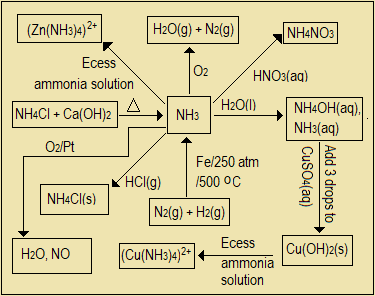
- Construct a flow diagram (reaction scheme) to represent the chemical reactions involving preparation and reactions of ammonia gas and its aqueous solution.
Answers to Questions 4.7 
Nitrogen and its Compounds
Extraction of nitrogen from the air: By fractional distillation of liquefied air.
Laboratory preparation of nitrogen gas
By chemical removal (elimination) of reactive components of air (CO2 and O2), then collecting nitrogen as a residual gas. Or by thermal decomposition of freshly prepared ammonium nitrite NH4NO2(s) → 2H2O(g) + N2(g)
It is insoluble in water, neutral, colourless, odourless, less dense than air, extinguishes burning and glowing wooden splints, and can be dried by passing it through concentrated sulphuric acid and collected by upward delivery. Combines directly with burning magnesium. 3Mg(s) + N2(g) → Mg3N2(s)
Uses of nitrogen
Manufacture of ammonia, nitrogenous fertilizers, preservative of biological specimen, achieving temperatures below -196 oC for study purposes, and treatment of skin and wart
Oxides of nitrogen
Nitrogen (I) oxide: Prepared in the laboratory by thermal decomposition of ammonium nitrate.
It is, colourless, denser than air, neutral, insoluble in water, has a faint sweet smell, relights a glowing splint, oxidizes metals and non-metals, forms a dark brown substance (FeSO4.NO) with iron (II) sulphate solution, and used as an oxidizer in racing cars.
Nitrogen (II) oxide: Prepared in the laboratory by reacting copper metal with dilute nitric (V) acid solution.
3Cu(s) + 8HNO3(aq) → 3Cu(NO3)2(aq) + 4H2O(l) + 2NO(g)
It is colourless, odourless, neutral, insoluble in water, extinguishes a burning and glowing splint, and of comparable density to air, oxidizes metals and non-metals, turns red-brown on exposure to air.
Nitrogen (IV) oxide: Prepared by reacting copper metal with concentrated nitric (V) acid.
Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2H2O(l) + 2NO2(g)
It is red-brown, has a chocking smell, extinguishes a burning and glowing splint, is acidic, denser than air, highly soluble in water forming nitric (III) acid (HNO2(aq)) and further to nitric (V) acid, and oxidizes metals and non-metals
Ammonia
Laboratory preparation of ammonia: Prepared by heating a mixture of calcium hydroxide and ammonium chloride solids. Ca(OH)2(s) + 2NH4Cl(s) → CaCl2(s) + 2H2O(g) + 2NH3(g)
It is colourless, less dense than air, highly soluble in water, the only ordinary basic gas, has pungent smell, dried using calcium oxide, burns with a blue flame, extinguishes a glowing splint, neutralizes acids, forms white fumes with hydrogen chloride gas, combines with calcium chloride (4NH3(g) + CaCl2(s) → CaCl2.4NH3(s)), used to manufacture fertilizers, smelling salts, and refrigerants.
Burning: 4NH3(g) + 3O2(g) → 9H2O(g) + 2N2(g); With HCl: NH3(g) + HCl(g) → NH4Cl(s)
Precipitates metallic ions: e.g. Ca2+(aq) + 2OH-(aq) → Ca(OH)2(s)
Reduces oxides of less reactive metals e.g. CuO. 3CuO(s) + 2NH3(g) → 3Cu(s) + 3H2O(g) + N2(g)
Catalytically oxidized to NO2 in presence of platinum. 4NH3(g) + 5O2(g) →
6H2O(g) + 4NO(g)
→
6H2O(g) + 4NO(g)
Industrial manufacture: N2(g) + 3H2(g) ⇄ 2NH3(g) at 200 atm, and 500 oC). Haber process
Used to manufacture fertilizers e.g. (NH4)2SO4, NH4NO3, (NH2)2PO4, Urea (NH2)2CO
Nitric (V) acid
Laboratory preparation of nitric (V) acid
KNO3(s) + H2SO4(l) → KHSO4(s) + HNO3(g)
Industrial manufacture of nitric (V) acid (Ostwald,s process)
Catalized oxidation of ammonia to NO, then with air to NO2; then dissolve the product in water in excess oxygen gas to form HNO3(aq). Distill the mixture.
Reactions of concentrated nitric (V) acid
Oxidizes metals and non-metals and itself reduced to NO2 and H2O
S(s) + 6HNO3(aq) → H2SO4(aq) + 2H2O(l) + 6NO2(g)
Fe(s) + 6HNO3(aq) → Fe(NO3)3(aq) + 3H2O(l) + 3NO2(g)
Thermally decomposes: 4HNO3(aq) 2H2O(l) + 4NO2(g) + O2(g)
2H2O(l) + 4NO2(g) + O2(g)
Reactions of dilute nitric (V) acid: Behaves typically as an acid.
Test for nitrates (brown-ring test)
FeSO4(aq) + NO(g) → FeSO4.NO(aq)
2H2O(l) + 4NO2(g) + O2(g) → 4HNO3(aq)
Protecting the environment from damage by nitric (V) acid
Apply lime to neutralize soil acidity; recycle and purify industrial wastes before releasing them into water bodies; convert all harmful substances to safe and environmentally friendly products; prevent leakage of harmful oxides.- (a) Test the gas with red and blue litmus. Also, pass it into a solution of copper (II) ions till in excess. Red litmus turns blue.
A blue precipitate which dissolves to form a deep blue solution when more of the gas is passed into a solution of copper (II) ions confirms
presence of ammonia.
(b) Waft the gas to smell it. Test the gas with a glowing splint. A faint sweet smell together with rekindling (relighting) of the glowing splint confirm that the gas is nitrogen (I) oxide.
(c) Expose the gas to the air. Also, pass it into a solution of iron (II) sulphate. Brown fumes on exposure to the air, and a brown solution with iron (II) sulphate indicates that the gas is nitrogen (II) oxide.
(d) Mix about 2 ml of iron (II) sulphate and 2 ml of the suspected nitrate solution in a test tube. Add a small volume of concentrated sulphuric (VI) acid carefully down the wall of the test tube. A brown-ring at the boundary between layers confirms the presence of nitrate ions. -
(Do likewise for nitrogen (I) oxide (N2O), nitrogen (II) oxide (NO), and nitrates.)