Answers section
Questions 5.3.2(b)
- Write the formula and name of each of the products of dehydration Solid G, Gas J, Gas L, Solid M.
- Write a complete equation for each of the dehydration reactions (b) to (e). Part (a) is already done below as an example.
CuSO4.5H2O(s)
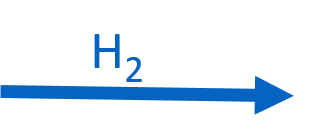 H2SO4(l) CuSO4(s) + 5H2O(l)
H2SO4(l) CuSO4(s) + 5H2O(l) - Identify what looks like white fumes in Figure 5.3.2(b), and explain why it is so thick.
- As shown in Figure 5.3.2(c) and the video, a relatively small amount of cane sugar produces a "large quantity" of black solid. Suggest an explanation for this.
- Explain why concentrated sulphuric (VI) acid is highly corrosive.
- Prepare a brief summary of the oxidation and dehydrating properties of concentrated sulphuric (VI) acid.
Answers to Questions 5.3.2(b) 
- Solid G: C, carbon; Gas J: C2H4, ethene; Gas L: CO, carbon (II) oxide; Solid M: C, carbon.
- (a) Done as an example
(b) C2H5OH(l) H2SO4(l) H2O(l) + C2H4(g)
H2O(l) + C2H4(g)
(c) C12H22O11 H2SO4(l) 12C(s) + 11H2O(l)
12C(s) + 11H2O(l)
(d) HCOOH(l) H2SO4(l) H2O(l) + CO(g)
H2O(l) + CO(g)
(e) nC6H10O5(s) H2SO4(l) 6nC(s) + 5nH2O(l)
6nC(s) + 5nH2O(l) - This is water vapour. It is thick because it is produced in large quantities as shown in equation 2(c), and the heat generated vapourizes it.
- The black solid is carbon. Removal of elements of water causes the carbon atoms in cane sugar to move further apart, so the solid becomes porous and occupies a much bigger volume.
- Concentrated sulphuric (VI) acid is highly corrosive due to its combined oxidizing and dehydrating properties.
- Concentrated sulphuric (VI) acid
Oxidation
Hot concentrated sulphuric acid oxidizes non-metals to their oxides, and it is itself reduces to sulphur (IV) oxide (SO2) and water (H2O).
S(s) + 2H2SO4(l) → 2H2O(l) + 3SO2(g)
C(s) + 2H2SO4(l) → 2H2O(l) + 2SO2(g) + CO2(g)
It oxidies metals to metal sulphates, and is itself reduced to sulphur (IV) oxide and water.
Mg(s) + 2H2SO4(l) → MgSO4(aq) + 2H2O(l) + SO2(g)
Cu(s) + 2H2SO4(l) → CuSO4(aq) + 2H2O(l) + SO2(g)
Generally: Metal + Concentrated sulphuric (VI) acid → Metal sulphate + Sulphur (IV) oxide + Water
Non-metal + Concentrated sulphuric (VI) acid → Non-metal oxide + Sulphur (IV oxide + Water
Dehydration High affinity for elements of water, hence a strong dehydrating agent.
Examples
Cane sugar (C12H22O11): C12H22O11 H2SO4(l) → 12C(s) + 11H2O(l)
Hydrated copper (II) sulphate: (CuSO4.5H2O): CuSO4.5H2O H2SO4(l) → CuSO4(s) + 5H2O(l)
Ethanol: (C2H5OH) C2H5OH(l) H2SO4(l) → H2O(l) + C2H4(g)
Methanoic acid (HCOOH): HCOOH(l) H2SO4(l) → H2O(l) + CO(g)
Wood or paper (-C6H10O5-)n: nC6H10O5(s) H2SO4(l) → 6nC(s) + 5nH2O(l)
The acid itself remains intact as H2SO4.
The acid is highly corrosive due to its combined oxidizing and dehydrating properties.
