CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Chemical Families and Patterns in Properties: Properties and Trends Across the Periodic Table
2.0 Chemical Families and Patterns in Properties
2.5 Properties and Trends Across the Periodic Table
What are the trends in physical and chemical behavior across periods? To answer this question, we will study Period 3 because it has many common or familiar elements available in the school laboratory (Figure 2.5).

Figure 2.5: Appearance of Period 3 elements
2.5.1 Trends in physical properties of elements in Period 3
The period begins with sodium, a soft metal. Softness decreases towards aluminium, with silicon being the hardest element in Period 3. The next element, phosphorus, is soft. Softness increases upto chlorine and argon which are gases and therefore the softest elements. Silicon (and Group IV) is therefore a transition point in behavior. These trends can be explained using the atomic structure (Figure 2.5.1).
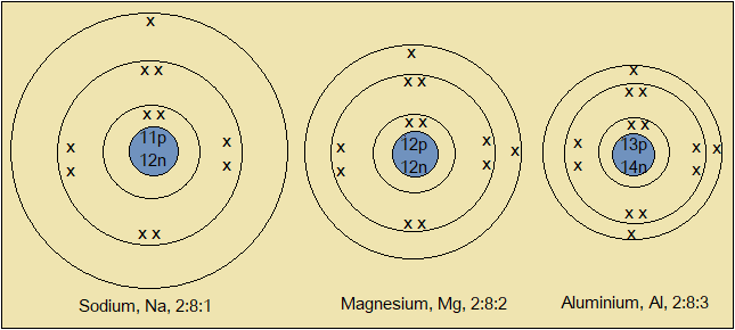
Figure 2.5.1: Atomic structures of some Period 3 elements
Questions 2.5.1
- What common feature of their atomic structures causes sodium, magnesium and aluminium to be in the same Period 3?
- What happens to the number of protons (+) in the nucleus as we move from left to right (sodium to aluminium) of the Periodic Table?
- How does this affect the strength of attraction of the nucleus on the electrons (-)?
- How would you expect this to affect the atomic radius?
- Sodium metal melts at 96oC, magnesium at 650oC, and aluminium at 660oC. Explain this trend in melting point.
- From previous experience, comment on the ability of the three metals to conduct electricity.
- Comment on the appearance of the three elements sodium, magnesium and aluminium.
Answers to Questions 2.5.1
As we move from left to right (sodium to aluminium), the number of energy levels remains the same. Yet the number of protons (+) in the nucleus increases. The increased nuclear charge (+) draws electrons (-) closer to the nucleus, reducing atomic radius. This trend continues upto chlorine, the smallest member (Figure 2.5.2).
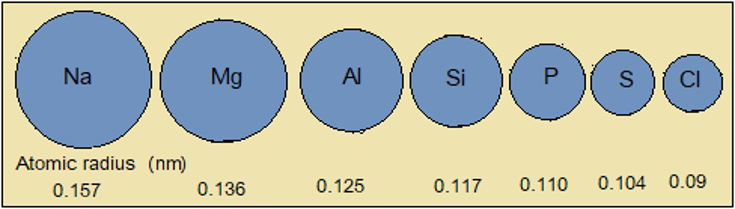
Figure 2.5.2: Trend in atomic radius across Period 3
NB: Figure 2.5.2 is simplified to show the trend in atomic radius only. It does not represent atomic structures of the elements.
2.5.2 Trends in chemical properties of elements in Period 3
Although the trends in atomic radius and atomic number are maintained throughout the period, this behavior is not true with chemical properties. This is because the period consists of metals, which react by losing electrons, and non-metals which react by gaining electrons. The elements can react among themselves, which is not common within other groups.
Questions 2.5.2(a)
- Which of the Period 3 elements react by losing electrons? Explain your answer.
- Which of the Period 3 elements react by gaining electrons? Explain your answer.
- Identify the element that reacts but is not likely to gain or lose electrons. Explain your answer.
- Name the stable element that does not react at all. Why is it unreactive? For the three metals sodium to aluminium, first ionization energy and reactivity depend on the ease with which their outermost electrons can be knocked off. First ionization energy is the amount of energy required to remove an electron from a neutral atom.
- Which of the three elements is expected to have the lowest first ionization energy? Explain your answer.
- Identify the least reactive element of the three. Explain your answer.
- Write the formulae of ions formed when sodium, magnesium and aluminium lose all their outermost electrons.
- How does ionic radius vary from sodium to aluminium?
- Write the valencies of all the elements in Period 3.
- Describe the change in valency as we move from sodium to argon.
Answers to Questions 2.5.2(a)
Unlike in groups, number of outer electrons and valency change across the Period. Atomic radius reduces instead of increasing. Sodium, magnesium and aluminium react by losing electrons, silicon neither loses nor gains but reacts by sharing electrons. Phosphorus, sulphur and chlorine react by gaining electrons but argon is stable.
Reactions of Period 3 elements with water and steam
Reactions of sodium with water, and magnesium with steam are already discussed under alkali- and alkali earth metals.
Aluminium, silicon, phosphorus, and sulphur hardly react with water. In fact phosphorus is safely stored in water.
As we learnt earlier under halogens, chlorine reacts with water to form hydrochloric acid (HCl) and chloric (I) acid (HOCl). Chloric (I) acid is unstable and when exposed to sunlight, it decomposes to form hydrochloric acid and oxygen gas. Chloric (I) acid causes bleaching of dyes (including litmus) by losing its oxygen atom to the dye.

Figure 2.5.3: Chlorine water exposed to sunlight
Questions 2.5.2(b)
- Write a balanced chemical equation for the reaction between chlorine and water.
- Write a balanced chemical equation for the decomposition of chloric (I) acid.
- Complete the following equation for the bleaching action of chloric (I) acid.
HOCl(aq) + Dye ⟶ Dye+O + ---
- What would you expect to observe during the time chlorine water (a mixture of HOCl and HCl) is exposed to sunlight?
- State and explain the observation that would be made when blue litmus paper is dipped into chlorine water that has been exposed to sunlight for some time.
Answers to Questions 2.5.2(b)
Reactions of Period 3 elements with oxygen
The burning of sodium and magnesium in oxygen are covered under the sections of alkali metals and alkali earth metals. All the other elements: aluminium, silicon, phosphorus, sulphur and chlorine react with oxygen to form oxides.
Questions 2.5.2(c)
Complete the following equations to represent reactions of Period 3 elements with oxygen.

Answers to Questions 2.5.2(c)
Aluminium usually forms a thin layer of oxide which protects it from further reaction with oxygen. But when polished and strongly heated, it reacts slowly with oxygen to form aluminium oxide which forms a protective layer around it and stops further reaction. This behavior, besides being a good conductor of heat, makes aluminium suitable for the manufacture of cooking utensils. Silicon burns at very high temperatures, while phosphorus and sulphur are soft solids which melt and burn readily.
Reactions of Period 3 elements with acids
Non-metals, that is silicon to chlorine do not react with acids. But like other metals, sodium, magnesium and aluminium react with acids to form salt and hydrogen gas. However, the reaction involving sodium must not be attempted.
Questions 2.5.2(d)
Write equations for the reactions between
- Magnesium and sulphuric acid
- Aluminium and hydrochloric acid
- Aluminium snd sulphuric acid
Answers to Questions 2.5.2(d)
Summary of trends across Period 3
- Atomic radius decreases from sodium in Group I to chlorine in Group VII.
- Melting point increases from sodium to silicon in Group IV then decreases generally towards argon in Group VIII.
- Valency and charge on ions increase from sodium to silicon and phosphorus, then decrease towards argon (Na+, Mg2+, Al3+, Si4, P3-, S2-, Cl-, Ar0)
- Reactivity decreases from sodium to silicon (Group IV) then generally increases towards chlorine.
Summary of trends down the Groups and across the Periods
Figure 2.5.4 is a summary showing how atomic radius varies down the groups and across the periods. This, together with the increase in atomic number down the group and across the periods causes gradation in physical and chemical properties of elements.
NB: There are a few exceptions to this trend, notably the noble gases. But even for these, atomic radius increases down the group.

Figure 2.5.4 Trends in atomic radii down and across periods
Project 2
Based on what you have learnt so far, prepare a presentation on any of the following sub-topics. Present it to your class or friends and prepare to answer their questions. You may include some new and relevant information; but do not overdo it.
- Akali metals
- Alkali earth metals
- Halogens
- Noble gases
- Period 3 elements
