CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Effect of an electric current on substances: Electrical conduction
5.0 Effect of an electric current on substances
5.1 Electrical conduction
Matter consists of electrical charges (negative (-) and positive (+)) because of the electrons and protons in it. But conduction of electricity depends on whether these charges are free to move about in the structure. Study Figure 5.1(a).

Figure 5.1(a) Structures of metals, ionic compounds and non-metals
Questions 5.1(a)
- Identify from A to E, the structures that conduct electricity.
- For each structure identified in Question 1, explain why it conducts electricity.
- For any one structure that does not conduct electricity, explain why it does not.
Answers to Questions 5.1(a)
Metals and graphite, whether solid or molten, have electrons which move about freely. In addition, ionic compounds in molten state or aqueous solutions have ions which move about freely. Therefore, they all conduct electricity. Non-metals and covalent compounds do not conduct electricity because they consist of neutral particles.
How do charges move naturally?
Natural movement of electrons in metals and graphite is similar to the random movement of molecules in liquids and gases. It occurs in all directions. The same behavior applies to ions in molten state or aqueous solutions of ionic compounds. But what is useful to us, as electrical energy, is movement that occurs in one direction.
How can we cause electrons and ions to flow in one direction?
Just like pressure difference is needed for air to flow in one direction (wind), we need electric potential difference (Voltage) for charges to move in one direction. This is provided by an electric cell (Figure 5.1(b)) or a battery of them.
Definitions
Study Figure 5.1(b) which shows how an electric cell is connected in a circuit to drive charges.
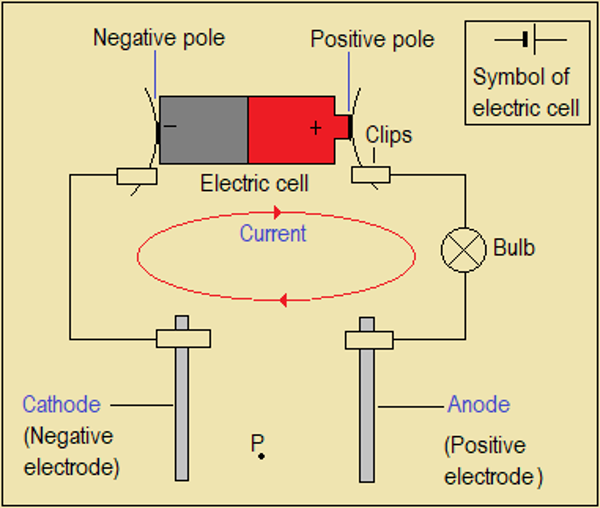
Figure 5.1(b) Circuit diagram to study effect of an electric current on substances
Electric Cell: The source of electricity. It drives electrons and negative ions in one direction.
Electrodes: Conductors connected to the negative or positive pole (end) of the electric cell.
Anode: The electrode connected to the positive pole of the cell. It is positive.
Cathode: The electrode connected to the negative pole of the cell. It is negative.
Anions: Negative ions (anode ions; i.e. ions attracted to the anode)
Cations: Positive ions (cathode ions; i.e. ions attracted to the cathode)
Circuit: The path along which electrical charges flow.
NB: Charges flow only when the circuit (path) is a complete loop. The red loop shown in Figure 5.1(b) assumes that the circuit is complete.
Electrodes are ordinary metals or graphite. They become anode or cathode when connected to the cell.
Questions 5.1(b)
Study Figure 5.1(b) above then answer the following questions.
- Suggest why an electric bulb is used. What can replace the bulb?
- P is an electrically charged particle. To which electrode will P move if it is positively charged?
- Solid electrodes conduct electricity by use of electrons. In which direction do electrons move in the circuit when completed: clockwise or anticlockwise?
- Name at least five (5) solid conductors of electricity.
Answers to Questions 5.1(b)
Substances which conduct electricity in solid state, also conduct electricity in molten state. They are elements with metallic structures, specifically metals and graphite. We can use this fact to identify metals and metallic structures.
