CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Chemical Bonding and Structure: Covalent bond
3.0 Chemical Bonding and Structure
3.4 Covalent bond
Consider two hydrogen atoms, each with one electron and lacking one electron to achieve the stable noble gas configuration.
Questions 3.4(a)
Can hydrogen atoms gain or lose electrons to each other? If not, how do they become stable?
Answers to Questions 3.4(a)
Hydrogen atoms become stable by pairing up to share their electrons (Figure 3.4(a)).

Figure 3.4(a): Covalent bond in hydrogen
The shared pair of electrons, represented by : make up the covalent bond shown. Two shared pairs means two bonds and so on. Covalent bonds are formed when non-metals combine with one another. Common covalently bonded substances include N2, O2, F2, Cl2, C, Si, P, S, Br2, I2, H2O, CO2, CO, NH3, HCl, SiO2, P2O5, P2O3, PCl3, PCl5, SO2, SO3, HBr, HI, and CH4. That is, covalent bond occurs within and between non-metallic elements.
Questions 3.4(b)
Draw dot and cross energy level diagrams to represent the covalent bonds in the following cases.
- Oxygen, O2
- Water, H2O
- Ammonia, NH3
- Carbon (IV) oxide, CO2
(O = 8; H = 1; N = 7; C = 6).
The figures in brackets are atomic numbers. To simplify the diagrams, show the outer energy levels only.
Answers to Questions 3.4(b)
Figure 3.4(b) shows the structures of some covalently bonded compounds, represented on dot and cross diagrams and simplified open structures. They are hydrogen chloride (HCl), sulphur (IV) oxide (SO2), and methane (CH4).
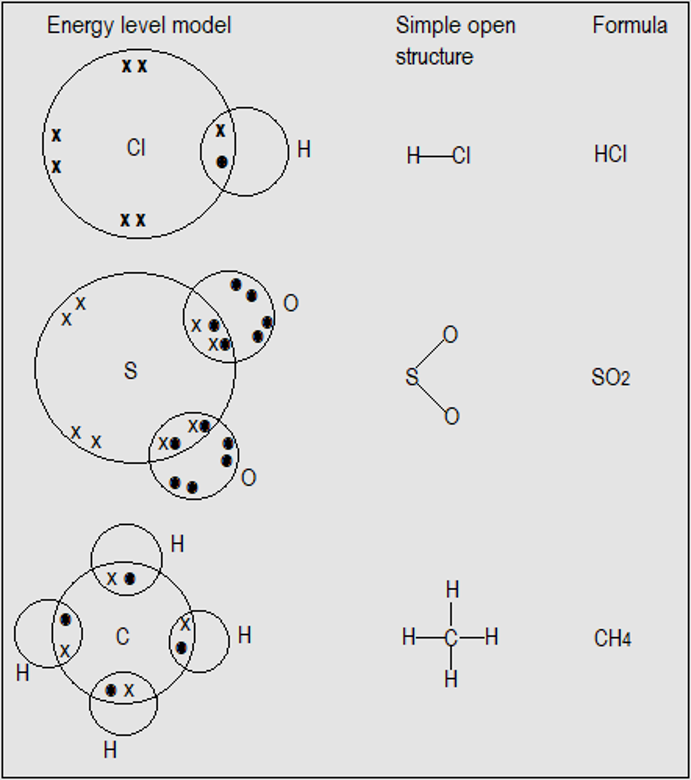
Figure 3.4(b): Structures of some covalently bonded compounds
This diagram shows different ways of representing the same compounds. Therefore, questions about structure normally indicate, sometimes indirectly, the representation required.
