CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Chemical Bonding and Structure: Metallic bond
3.0 Chemical Bonding and Structure
3.8 Metallic bond
What do we know about metals?
- They are hard solids, with high melting points.
- They conduct electricity, even as solids.
These properties give us a clue about the structure of metals.
Let us consider an atom of sodium metal, whose electronic configuration is 2:8:1.
Questions 3.8(a)
- How can a sodium atom become stable? Explain your answer.
- If electrons are "lost", where do they go?
- The positively charged nuclei in sodium atoms repel one another. Yet sodium, like other metals, does not bulge out. How can the "lost" electrons help in holding the positive nuclei together?
- In this arrangement, how many nuclei and electrons do you expect to be involved?
- How would you describe the resulting structure?
- What enables this structure to conduct electricity?
- Suggest a name for the bonds in sodium and other metals.
Answers to Questions 3.8(a)
In metallic bonding, each atom gives up its outer electron to achieve the stable noble gas configuration. Such electrons form a pool of mobile negative charge, dispersed between the positive ions. The negative charge and positive ions attract each other, thereby holding the structure together (Figure 3.8).
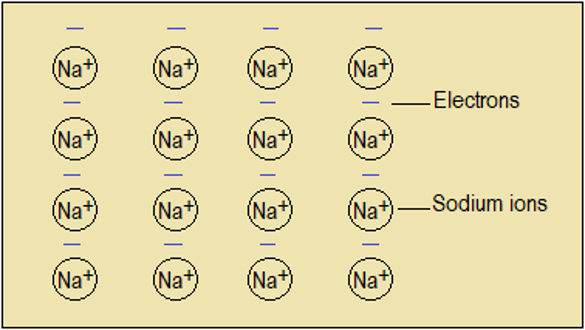
Figure 3.8: Metallic structure of sodium metal
Observe the animation of movement of electrons in a metallic structure, below.
courtesy Youtube-Metallic bonding by Chemistry Channel)NB: The number of ions equals number of electrons shown because each sodium atom releases its one outer electron. Electrons move in the vertical spaces between ions as well.
Because these electrons are no longer confined to individual atoms, they are said to be delocalized. Delocalized electrons also carry heat from one point to another, making metals good thermal conductors as well. Thermal means, to do with heat.
The giving up of electrons in chemical reactions is a property of metals; so the bonds formed within a metal are called metallic bonds. Where more electrons are used, the bond becomes stronger, melting point is higher, and so are electrical and thermal conductivity. Metallic bonds extend in all directions (in a repeated pattern), involving very many ions; so it leads to a giant metallic structure.
Questions 3.8(b)
- Draw labelled diagrams to represent the structures of magnesium and aluminium metals (Mg = 12; Al = 13).
- How does an ion differ from a nucleus?
Answers to Questions 3.8(b)
When drawing the metallic structure, the number of electrons in the mobile pool should equal the total number of positive charges or unbalanced protons in the ion. For example, for 12 ions of aluminium, there are 36; that is, 3x12 electrons in the pool, because each aluminium atom releases 3 electrons. And because ions are literally immersed in a pool or sea of electrons, it would not be accurate to represent bonds using a ball-and-stick model.
Properties of metals
- They generally have giant metallic structures.
- They generally have high melting and boiling points.
- They conduct electricity in solid, liquid (mercury) and when molten. This is the property used to identify metals.
Questions 3.8(c)
- How does metallic bond differ from ionic bond?
- Give two reasons why melting point increases from sodium to aluminum.
- Aluminium is a better conductor of electricity than sodium and magnesium. Explain this.
- Explain why sodium (or potassium) is considered as a giant structure, yet it is soft, with a low melting point.
- Explain why sodium has a low melting point yet it has a giant structure.
Answers to Questions 3.8(c)
Summary about substances with ionic, covalent, and metallic structures
Table 3.8(a): Summary about structures

NB: The properties in bold are used to identify the structures.
Questions 3.8(d)
Table 3.8(b) gives some information about substances Q, R, T, X, and Z. Use the information to answer the questions that follow.
Table 3.8(b) Some physical properties of Q, R, T X and Z

Identify the substance(s) with the following properties
- Giant metallic structure
- Simple molecular structure
- Giant (atomic) covalent structure
- Giant ionic structure
- A gas at room temperature
- A liquid at room temperature
- Having covalent bonds
Answers to Questions 3.8(d)
