CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Carbon and some of its compounds: Carbon cycle
6.0 Carbon and some of its compounds 
6.7 Carbon cycle
Some processes absorb carbon (IV) oxide, while others produce or release it to the atmosphere. We need a balance between the amounts produced and absorbed to have a safe environment. Major absorbers and producers include the following.
Photosynthesis: Green plants absorb and use carbon (IV) oxide to make food.
Sea water: Sea water absorbs some carbon (IV) oxide to form carbonic acid (H2CO3). When temperature increases, some carbonic acid dissociates back to CO2 (and H2O).
Combustion: Burning fossil and wood fuels produce carbon (IV) oxide (and water).
Natural decay: Dead animals and plants decay (decompose) to humus then slowly to fossil fuels over millions of years, under high temperatures and pressure. In this form, carbon has no effect on climate till it is burnt. That is, fossil and humus are carbon sinks.
Respiration: Plants (at night) and animals produce carbon (IV) oxide to the air.
A diagram showing the relationships between producers and absorbers of carbon (VI) oxide is called Carbon Cycle (Figure 6.7). At the moment, the global problem is how to reduce the amount released, increase the amount absorbed, or create carbon sinks).
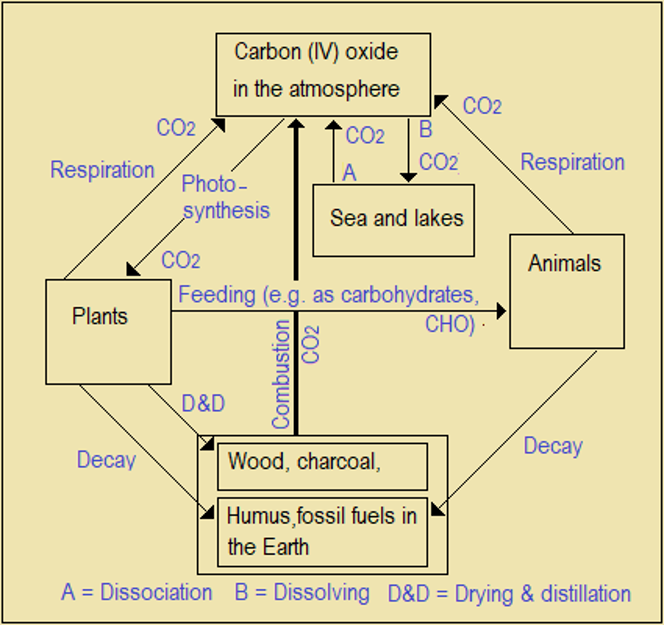
Figure 6.7 Carbon Cycle (Bold arrow shows the greatest single producer of CO2)
Questions 6.7
- How does the pH of sea water change as the amount of carbon (IV) increases and why?
- What would be some of the effects of pH change of sea water?
- Referring to the carbon cycle in Figure 6.7, suggest two possible methods which may be used to reduce the amount of carbon (IV) oxide already in the atmosphere or being released to it.
Answers to Questions 6.7
Project 6
Read more about carbon, carbon farming, carbon sink, global warming and climate change. Then (or otherwise) design a Project to help your school or community reduce the amount of carbon (IV) oxide released to the atmosphere. Share this with your teacher and present it as creative work.
