CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Structure of the Atom, and the Periodic Table: Chemical Formulae
1.0 Structure of the Atom, and the Periodic Table
1.8 Chemical Formulae
In Section 1.7 (Ion Formation) we learnt that unstable atoms react by losing or gaining electrons to complete their energy levels and become stable. We can therefore easily determine a chemical formula by considering how energy levels can be completed. We will do this through examples.
1.8.1 Chemical formulae of reactive gaseous elements
Open the pencast on formulae of gaseous elements.
Study Figure 1.8.1 which shows hydrogen atoms and how they combine.
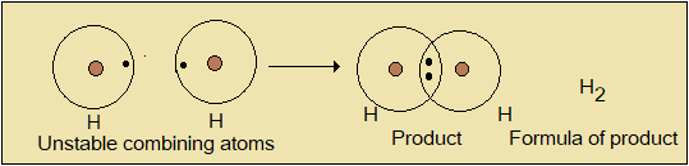
Figure 1.8.1: Formula of hydrogen
Single hydrogen atoms have incomplete energy levels; so they are unstable. When two such atoms come together, the energy level in each becomes complete, with 2 electrons so the product has the formula, H2. A third hydrogen atom is not required.
Hydrogen therefore exists as pairs of atoms, called diatomic molecules. Many other gaseous elements such as nitrogen (N2), oxygen (O2), fluorine (F2), and chlorine (Cl2) exist as diatomic molecules.
Solid elements are represented by their chemical symbols as though they were single atoms. Examples are lithium (Li), carbon (C), sodium (Na), magnesium (Mg), aluminium (Al), phosphorus (P), sulphur (S), potassium (K), and calcium (Ca).
1.8.2 Chemical formulae of compounds
Study the following examples presented on diagrams to show how to arrive at chemical formulae of compounds.
Example 1: Formula of lithium hydride
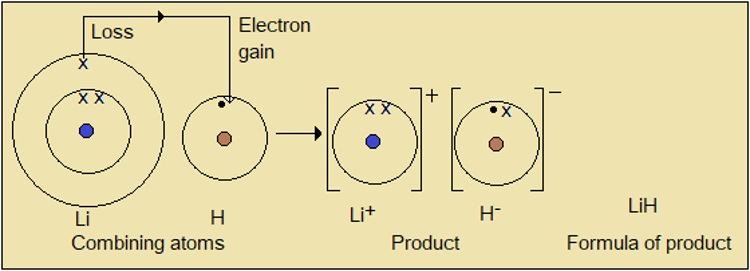
Figure 1.8.2: Formula of lithium hydride
One (1) atom of lithium combines with one (1) atom of hydrogen (H); so the chemical formula of the product is Li1H1, which is simplified as LiH. Note that the combining atoms have the same number of lone or unpaired electrons, which explains the ratio 1:1. Also, in compounds where there are metals, the metal comes first in the formula.
Where only non-metals are involved, atoms share rather than lose or gain electrons (Example 2).
Example 2: Formula of water
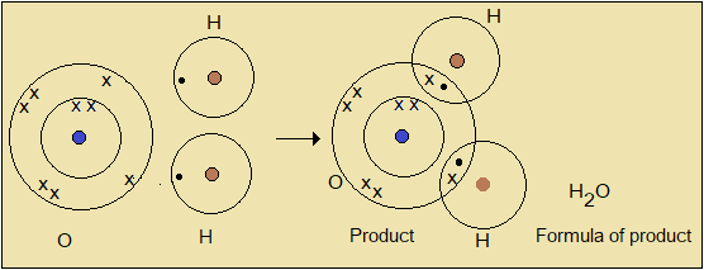
Figure 1.8.3: Chemical formula of water
Oxygen atom has two (2) lone electrons while hydrogen has one (1). Two hydrogen atoms are therefore required to satisfy one oxygen atom. This leads to the familiar formula of water, H2O. Notice that in the structure of H2O, oxygen and hydrogen atoms have complete energy levels with 8 and 2 electrons respectively; so they are stable.
From these examples, we notice that chemical formula of a compound (made up of different elements)
- Depends on number of lone electrons (valency) in the atoms
- Take the form XyYx
(where X and Y are symbols of the elements; y is the valency of Y; x is the valency of X)

Figure 1.8.4: General chemical formula
NB: In the formula XxYy, x and y must be in the simplest ratio. For example, X2Y4 becomes XY2.
We have used Figures 1.8.1 to 1.8.3 to understand why reactive gaseous elements exist as paired atoms and how valency comes about. Valency is the combining power of an element or atom. It equals the number of lone electrons and normally ranges between 1 and 4.
Otherwise, if valency is known, we can use Figure 1.8.4 directly to arrive at a chemical formula. Table 1.8.1 shows the valencies of some common elements and ions.
Table 1.8.1: Valencies of some common elements and ions
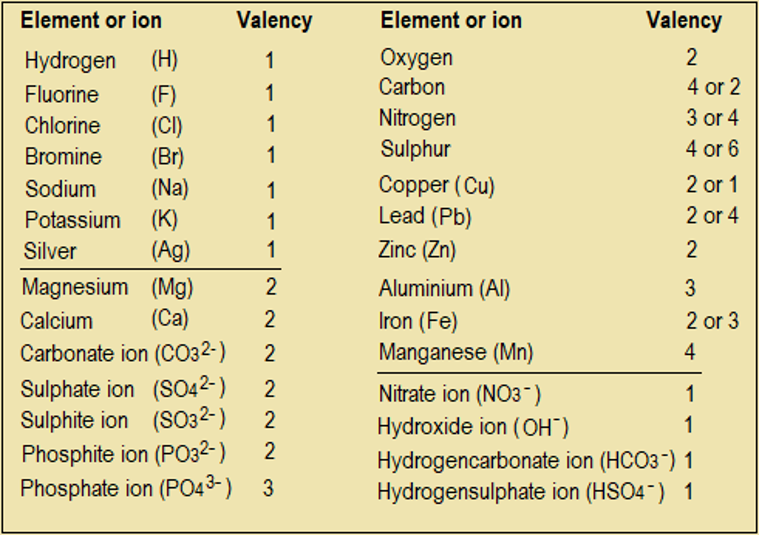
NB: The charge on an ion equals its valency.
Following the guide in Figure 1.8.4, the formula of zinc nitrate, for example, is Zn(NO3)2.
NB: Remove the charge on an ion once it combines with another, because it becomes neutralized.
Questions 1.8 
Use the valencies given in Table 1.8.1 to write the chemical formulae of the compounds named in Questions 1 and 2.
-
- Hydrogen chloride (same formula as hydrochloric acid)
- Potassium hydroxide
- Magnesium chloride
- Calcium carbonate
- Calcium chloride
- Aluminium oxide
- Sodium sulphate
- Potassium nitrate
- Ammonium nitrate
- Carbon (IV) oxide
- Zinc sulphate
- Copper nitrate
- Ammonium phosphate (V)
- Silver nitrate
-
- Lead (II) oxide
- Lead (II) nitrate
- Iron (II) oxide
- Iron (III) oxide
- Iron (II) sulphate
- Copper (II) oxide
- Copper (I) sulphide
- Manganese (IV) oxide
- Sulphur (VI) oxide
- Sulphur (IV) oxide
NB: The values given in Roman numbers are the valencies of the metals involved.
Answers to Questions 1.8
