CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Carbon and some of its compounds: Carbon (IV) oxide
6.0 Carbon and some of its compounds
6.3 Carbon (IV) oxide
6.3.1 Preparation of carbon (IV) oxide
Questions 6.3.1(a)
What are the general products of reaction between acids and carbonates?
Answers to Questions 6.3.1a
We use our knowledge of reactions between acids and carbonates to prepare carbon (IV) oxide. This, we do by selecting and reacting a suitable carbonate (e.g. CaCO3) and dilute acid (e.g. HCl(aq)).
Set-up
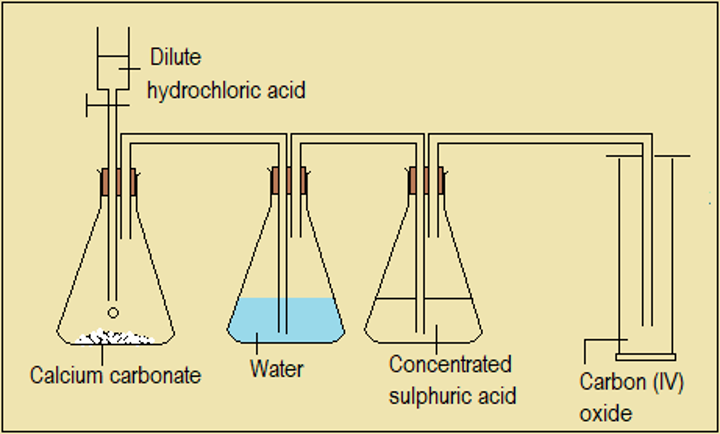
Figure 6.3.1 Preparation of carbon (IV) oxide
Water (sometimes sodium hydrogencarbonate solution) is used to absorb hydrogen chloride spray from hydrochloric acid.
Questions 6.3.1(b)
- State the function of concentrated sulphuric acid in this set-up. Name an alternative substance for concentrated sulphuric acid.
- Write equation for the reaction between hydrochloric acid and calcium carbonate.
- Name the method of delivery shown in the diagram.
- Explain why this method of delivery is suitable for collecting carbon (IV) oxide.
- What is the effect of the gas on wet blue litmus paper? What does this show?
- What is the effect of inverting the gas jar over the flame of a burning candle?
- Normally carbon (IV) oxide does not support burning. But ignited magnesium continues to burn in carbon (IV) oxide with a very hot flame. Suggest a reason for this. What are the other observations? Identify the other products observed.
Answers to Questions 6.3.1b
Although all dilute acids produce carbon (IV) oxide when reacted with carbonates, not all of them are suitable. Dilute sulphuric acid, for example, forms an insoluble (only slightly soluble) calcium sulphate which stops the reaction immediately by coating the unreacted carbonate. Note that calcium chloride (CaCl2) is soluble. For the same reason, carbonates of lead and barium should not be reacted with sulphuric acid.
6.3.2 Reactions of carbon (IV) oxide
We have already observed some important reactions of carbon (IV) oxide in Section 6.3.1.
Questions 6.3.2
Write equations to represent the following reactions of carbon (IV) oxide.
- Carbon (IV) oxide slightly dissolves in water to form carbonic acid (H2CO3) which is a weak acid and turns blue litmus pink.
Equation:
- Carbon (IV) oxide reacts with calcium hydroxide solution to form a white precipitate, which is calcium carbonate.
Equation:
- Excess of carbon (IV) oxide causes the carbonate to dissolve by forming calcium hydrogencarbonate, Ca(HCO3)2. Water also takes part in this reaction.
Equation:
- In the same manner, carbon (IV) oxide reacts with sodium hydroxide solution to form sodium carbonate (Na2CO3). But, unlike calcium carbonate, this is soluble; so it is in solution.
Equation:
- With excess of carbon (IV), sodium carbonate (Na2CO3) reacts to form sodium hydrogencarbonate solution (NaHCO3).
Water also takes part in this reaction.
Equation:
- Carbon (IV) oxide reacts with burning magnesium to form a white solid, which is magnesium oxide, and carbon as black specks.
Equation:
Answers to Questions 6.3.2
Note that the formation of white precipitate with calcium hydroxide, used to test for carbon (IV) oxide, occurs only during the first few minutes of bubbling the gas through the solution. On further bubbling of the gas (excess of CO2), the precipitate dissolves into a colourless solution (calcium hydrogencarbonate), a two-stage reaction.
Because of the two-stage reactions with carbon (IV) oxide gas, sodium hydroxide solution is a good absorber of the gas. Potassium hydroxide solution (KOH(aq)) reacts with carbon (IV) oxide in the same manner as NaOH.
2KaOH(aq) + CO2(g) ⟶ K2CO3(aq) + H2O(l)
K2CO3(aq) + CO2(g) + H2O(l) ⟶ 2KHCO3(aq)
6.3.3 Properties of carbon (IV) oxide
- Colourless and odourless gas
- Denser than air
- Extinguishes a burning splint or candle (does not support combustion)
- Reacts with/dissolves in sodium hydroxide solution to form sodium carbonate and sodium hydrogencarbonate (baking powder)
- Sublimes (with a cooling effect)
- Forms a white precipitate with calcium hydroxide solution, which dissolves in excess of the gas. This is the usual test for the gas.
6.3.4 Uses of carbon (IV) oxide
Questions 6.3.3
From the properties stated, suggest 3-4 uses of carbon (IV) oxide.
Answers to Questions 6.3.3
- Carbon (IV) is used as a fire extinguisher. Why? Because it is denser than air and does not support combustion, carbon (IV) oxide acts as a blanket over the fire, cutting off oxygen to stop the fire.
- Substances which readily evaporate or sublime do so by absorbing heat; so they have a cooling effect. Therefore solid carbon (IV) oxide, which sublimes, is used as a refrigerant.
- It is used to make carbonated fizzy drinks. The gas is dissolved in water under pressure to make soda water, which helps in digestion and preservation. During use, some of the gas escapes as bubbles. Watch the video of a fizzy drink below.

Figure 6.3.4(a) Fire extinguisher
When extinguishing a fire, the jet from the gas canister should be directed at the base of the fire. If directed above the flame, it will heat up, expand, become less dense are rise without putting out the fire.
(courtesy Youtube-Fizzy drink, carbonated drink by Joseph Rabari)
