CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Structure of the Atom, and the Periodic Table: Isotopes
1.0 Structure of the Atom, and the Periodic Table
1.4 Energy levels and electron arrangement
Previously, we learnt that electrons were negatively charged.
Questions 1.4(a)
- What would happen between electrons if they are very close to one another?
- What would happen between an electron and the positively charged nucleus?
-
- Describe the path taken by the ball as it moves.
- What kind of motion is this? (Hint: motion that causes seasons on the Earth).
- What happens when the swirling is stopped?
- What causes the behavior in 3(c)?
- What does the spring or elastic represent in this demonstration?
- When an object stops revolving or moving in a circle, it falls to the centre of the circle. True or False?
- During motion of electrons around the nucleus, they do not move to the nucleus; yet they are attracted there. What is keeping electrons away from the nucleus?
Observe the demonstration alongside Figure 1.4.1, to see why electrons do not fall to the nucleus even though they are attracted there.
(courtesy Youtube-Why electrons do not fall to the nucleus by Joseph Rabari)
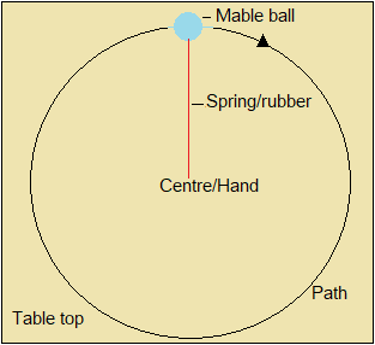
Figure 1.4.1: Marble ball whirled on a spring or elastic
Answers to Questions 1.4(a)
Attraction occurs between electrons and nucleus, because they have opposite charges. We would therefore expect electrons to be together with protons in the nucleus. Their being apart suggests that electrons move around the nucleus continuously. This is because, as we have seen in the demonstration, motion around the centre keeps an object away from the centre (Figure 1.4.1).
How do electrons move around the nucleus?
Do you know that there are times when the moon is closer to the earth, and the earth is closer to the Sun than at other times as they move? Electrons also change their distance continuously as they move around (revolve) the nucleus, but much faster. Watch the pencast of how electrons move around the nucleus.
(courtesy Youtube-How electrons move around the nucleus by Joseph Rabari)
We therefore do not place electrons on orbit or energy level but slightly below it as we did in Figures 1.1.1 to 1.3.1. This is to mean that they can be found anywhere between the nuceus and the orbit.
How do the numbers of protons and electrons in an atom compare?
Think for a moment. Materials around us such as paper and cloth do not attract one another till they are charged, say, by rubbing. They are electrically neutral, and so are their atoms. That is, each atom has the same number of protons and electrons; so the positive and negative charges cancel out or neutralize each other (Table 1.4.1).
Table 1.4.1: Equal numbers of protons and electrons

Do all electrons in an atom occupy the same distance from the nucleus?
Let us find out, guided by Questions 1.4(b).
Questions 1.4(b)
- What is the electrical charge on each electron?
- Would all electrons crowd together at a point? Explain your answer.
- Can all electrons be at the same distance from the nucleus? Explain your answer.
Answers to Questions 1.4(b)
Because of like charges and repulsion between electrons, some of them are pushed to a greater distance from the nucleus; so they move within different orbits, called energy levels.
Which energy levels (or electrons) have more energy: those nearer or farther from the nucleus?
Observe the demonstration of energy level and note what happens to radius of the path as speed increases. Then answer Questions 1.4(c).
(courtesy Youtube-Demonstration of energy level by Joseph Rabari)
Questions 1.4(c)
- What happens to radius of the path as speed increases (or movement becomes faster)?
- How does speed relate to energy?
- Which energy levels have less energy: those nearer or farther from the nucleus?
Answers to Questions 1.4(c)
Energy levels are labelled K, L, M, N and so on, moving away from the nucleus. The energy level, K, that is closest to the nucleus corresponds to the least energy. Therefore, electrons occupying this level have the least energy.
Do all energy levels hold (accommodate) the same number of electrons?
Think of it. K is smaller, with less space; so it holds fewer electrons than L. L, M, and N can hold upto 8 electrons each, leading to the pattern 2.8.8.8, called the octet rule, because of 8 (Table 1.4.2).
Table 1.4.2 Distribution of electrons on energy levels
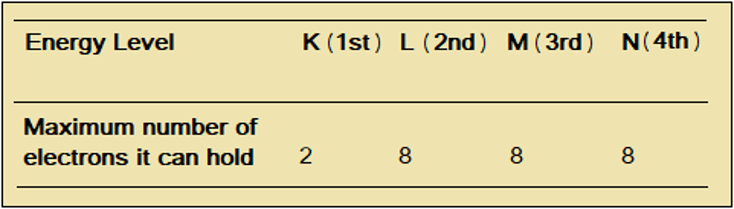
We can use the octet rule to distribute upto 20 electrons in the energy levels K to N. Distribution of electrons as 2:8:8:x, is called electronic configuration or electron arrangement.
There are two ways to represent electron arrangement, namely:
- Writing it in the form 2:x:y:z (i.e. electronic configuration) or
- Drawing dot (.) or cross (X) energy-level model, sometimes called a shell model.
Questions 1.4(d)
Write the electronic configurations of elements with the following atomic numbers.
- 14
- 18
Answers to Questions 1.4(d)
Suggested procedure to distribute electrons by drawing
- Draw the nucleus and first energy level, K, around it. If there is only one electron (for an element with atomic number 1), mark it as X, -, or dot within K. If there are 2 electrons, pair them up within K.
- If there are more than 2 electrons, place a pair within K. Carry the rest to energy level, L, distributing them one by one at the four positions: left, top, right, and down. If there are still more electrons, begin pairing them upto a maximum of 4 pairs (8).
- If there are still more electrons, move to energy level, M, and repeat Step 2 of the procedure.
NB: Number of protons (atomic number) and electrons must be equal. Atomic numbers are normally given when required for use. Electrons can pair up if one of them rotates clockwise and the other, unticlockwise.
Watch the pencast of how to draw dot and cross atomic structures.
(courtesy Youtube-Drawing atomic structures by Joseph Rabari)
Questions 1.4(e)
Draw the atomic structures of elements with the following atomic numbers.
- lithium (3 protons, 4 neutrons)
- chlorine (17 protons, 18 neutrons)
Answers to Questions 1.4(e)
Questions 1.4(f)
- Write the electronic configuration for each of the following elements (atomic numbers given in brackets).
- Nitrogen, N (A = 7)
- Oxygen, O (A =8 )
- Neon, Ne (A = 10)
- Magnesium, Mg (A = 12)
- Phosphorus, P (A = 15)
- Chlorine, Cl (A = 17)
- Calcium, Ca (A = 20)
- Draw the atomic structure for each of the elements (a) to (f) in Question 1. Ignore the number of neutrons.
- An atom Q has 19 protons and 20 neutrons. Write its electronic configuration.
Answers to Questions 1.4(f)
