CHEMISTRY FORM 2
- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis
- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Structure of the Atom, and the Periodic Table: Relative Atomic Mass and Isotopes
1.0 Structure of the Atom, and the Periodic Table
1.6 Relative Atomic Mass and Isotopes
What is relative atomic mass?
In Section 1.3, we learnt that an element with isotopes (atoms with the same number of protons but different numbers of neutrons) has different mass numbers. How can we have a single mass value for each element?
First, we should have a mass scale on which we can compare masses, because we are not dealing with actual values, which are very small. Scientists have agreed on a scale on which carbon-12 isotope is assigned a mass value of exactly 12 units, because it has a mass number of 12 (6 protons + 6 neutrons).

Figure 1.6: Carbon-12 relative mass scale
The mass of an atom on this scale is called Relative Atomic Mass (R.A.M). Relative Atomic Mass is the mass of an atom on a scale where carbon-12 isotope weighs exactly 12 units
.Whereas mass number (sum of protons and neutrons) is a whole number, R.A.M is an average of the masses of various isotopes of an element; so it can be in decimals. It is a single value that represents an element. Certainly, one unit on this scale, called atomic mass unit (a.m.u), equals 1/12 of mass of carbon-12 isotope.
To determine R.A.M for elements with isotopes
Suppose among 40 atoms of an element, Q, 15 weigh 60 units each (mass number of 60) and 25 weigh 50 units each (mass number of 50). What is the R.A.M of Q.
Solution: We are to work out the average mass, called weighted mean.
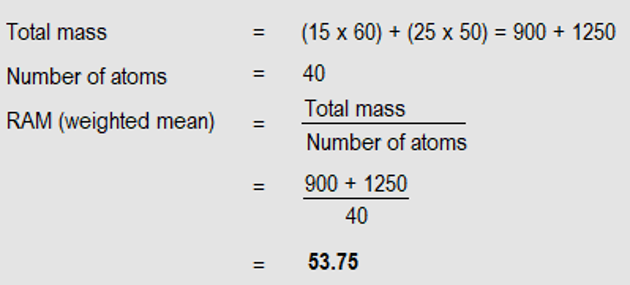
NB: As a relative quantity, R.A.M has no units.
Calculating R.A.M, involves working out the weighted mean. That is, we consider the number (in this case 15 and 25) and isotopic masses (in this case 60 and 50). Sometimes, the numbers are given as percentages; but the calculations are the same.
Questions 1.6
- A sample of chlorine consists of 25% chlorine-37 and 75% chlorine-35 isotopes. Determine the relative atomic mass (R.A.M) of chlorine.
- An element consists of two isotopes of mass number 12 and 14. The percent abundance of the heavier isotope is 5%. Determine the relative atomic mass (R.A.M) of the element.
Answers to Questions 1.6
NB: The percentages are also called percent abundances.
