CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Alkali Earth Metals (Group II elements), Physical properties of alkali earth metals, Chemical properties of alkali earth metals,Chemical formulae of some alkali earth metal compounds
2.0 Chemical Families and Patterns in Properties
2.2 Alkali Earth Metals (Group II elements)
Alkali earth metals are elements in Group II of the Periodic Table. That is, beryllium, magnesium, calcium, barium and others.
Beryllium (Be)
Magnesium (Mg)
Calcium (Ca)
We will find out why they are referred to as alkali earth metals.
2.2.1 Physical properties of alkali earth metals
Figure 2.2.1 shows the appearance of alkali earth metals.

Figure 2.2.1: Appearance of alkali earth metals
Observe the video demonstration of physical properties of alkali earth metals below. In addition, study the atomic structures of the same metals presented in Figure 2.2.2
NB:Observe the appearance of the metals (first part) only.
(courtesy Youtube-Alkaline Earths - Group 2 Properties by Angles and Acid )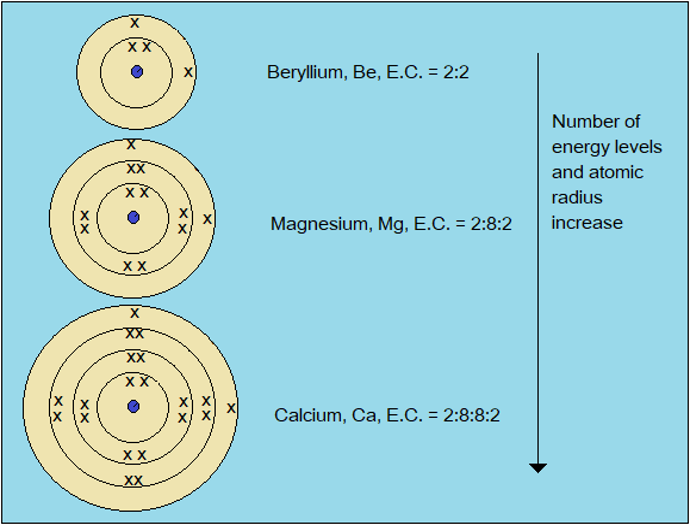
Figure 2.2.2:Atomic structures and configurations of alkali earth metals
From the video demonstration and Figures 2.2.1 and 2.2.2, answer Questions 2.2(a).
Questions 2.2(a)
- Describe the texture (hardness or softness) of alkali earth metals. How does it differ from the texture of alkali metals?
- How would you expect hardness and melting point to change as we move from beryllium to calcium and lower? Explain you answer.
- How would you expect the melting point of beryllium to compare with that of magnesium?
- Why are alkali earth metals placed in the same Group (II) of the Periodic Table?
- Describe the appearance of fresh surfaces of alkali earth metals.
- Do alkali earth metals conduct electricity?
Answers to Questions 2.2(a)
Atomic radius of alkali earth metals increases down the group (Table 2.2). This is because of the increase in number of energy levels. As in the case of alkali metals, this leads to a general decrease in melting point as atoms become less closely packed and therefore weakly held together.
Table 2.2 Atomic radii and melting points of alkali earth metals
| Element | Atomic radius (nm) | Melting point (oC) |
| Beryllium | 0.089 | 1280 |
| Magnesium | 0.136 | 650* |
| Calcium | 0.174 | 850 |
| Strontium | 0.210 | 789 |
2.2.2 Chemical properties of alkali earth metals
Observe the video demonstration of chemical properties of alkali earth metals below and answer Questions 2.2(b).
(courtesy Youtube-The Chemistry of Group 2 by Rugby School Chemistry)
Questions 2.2(b)
Refer to Figure 2.2.1 (pictures of alkali earth metals) as you answer the following questions.
- Which of the elements forms ions least readily (is least reactive). Explain your answer.
- What is the charge (type and amount) on the ions of alkali earth metals?
- In what ways do the ions of alkali earth metals differ among themselves?
- Write the formulae of ions formed by beryllium, magnesium and calcium.
- Write the electronic configurations of beryllium, magnesium and calcium ions.
- What is the valency of alkali earth metals?
Answers to Questions 2.2(b)
Reaction with steam
Observe the demonstration of reaction between magnesium and steam below, then answer Questions 2.2(c).
(courtesy Youtube-Reaction of magnesium with steam by liyauman)
Questions 2.2(c)
- Describe what is observed when
- steam is passed over heated magnesium ribbon.
- the solid product is mixed with water, shaken and tested with litmus.
- Write a chemical equation for the reaction between
- magnesium and steam
- calcium and steam
- the product in 1(a) and water
- Suggest a reason why Group II elements are referred to as alkali earth metals.
Answers to Questions 2.2(c)
Group II elements react with steam to form metal oxides (BeO, MgO, CaO) and hydrogen gas (H2). Their metal hydroxides [(Be(OH)2 , Mg(OH)2 , Ca(OH)2)] dissolve slightly in water to form alkaline solutions, which turn red litmus blue.
Generally compounds of Group II elements are largely insoluble; so they naturally occur below the earth as solid ores and minerals. Accordingly, they are referred to as alkali earth metals.
Caution: Reaction between calcium and steam should not be demonstrated because it is explosive and dangerous.
Reaction with oxygen
Observe the demonstration of reaction between alkali earth metals and oxygen gas.
NB: Forward and watch from the 4.40th to 8th minute.
(courtesy Youtube-Reactions of Alkali Metals with Oxygen by Mindset)Questions 2.2(d)
- Describe the observations made when a burning piece of magnesium is lowered into a jar full of dry oxygen gas.
- Write chemical equations for the burning of
- beryllium in oxygen
- magnesium in oxygen
- calcium in oxygen
- Alkali earth metals react with steam, as well as oxygen gas. What is common in the products of both reactions?
Answers to Questions 2.2(d)
Alkali earth metals burn with their characteristic bright colors to form metal oxides, which are all white powders. Magnesium burns with a bright white flame and calcium with a bright red flame. Left exposed, these metals slowly react with oxygen of the air and turn into metal oxides (loose white powder), similar to the free end of the magnesium ribbon in Figure 2.2.1.
Reaction with chlorine
Observe the demonstration, reaction between alkali earth metals and chlorine gas.
(courtesy Youtube-Burning Magnesium in Chlorine Gas by Jackson Page)
Questions 2.2(e)
- Describe the observations made when a burning piece of magnesium is lowered into a jar full of dry chlorine gas.
- Write chemical equations for the burning of
- beryllium in chlorine gas
- magnesium in chlorine gas
- calcium in chlorine gas
Answers to Questions 2.2(e)
Alkali earth metals burn in chlorine gas with bright flames to produce metal chlorides, which are white solids. This reaction becomes more vigorous down the Group as we move from beryllium to calcium and beyond.
Reaction with dilute acids
Observe the demonstration on reactions of alkali earth metals with dilute acids below.
(courtesy Youtube-Magnesium ribbon in hydrochloric acid by Ms. Wall's Classroom)
Questions 2.2(f)
- Describe the observations made when magnesium metal is added to dilute hydrochloric acid.
- Write a chemical equation for the reaction between
- beryllium and hydrochloric acid
- magnesium and hydrochloric acid
- calcium and hydrochloric acid
Answers to Questions 2.2(f)
Alkali earth metals react vigorously with dilute acids to form salt and hydrogen gas. Remember, BeCl2, MgCl2, and CaCl2 are also salts. The vigor of this reaction (reactivity) increases down the group from beryllium to calcium and further to strontium (Sr), barium (Ba) and radium (Ra). That is, calcium is more reactive than magnesium, which is more reactive than beryllium, and so on (Ca>Mg>Be).
2.2.3 Chemical formulae of some alkali earth metal compounds
As we learnt previously, chemical formulae are determined by valencies of elements or groups of the elements combined in the compound.
We have already practiced writing chemical formulae in Section 1.8. Use the same skills to complete Table 2.2.3.
Questions 2.2.3
- Complete Table 2.2.3 to show the formulae of some compounds of alkali earth metals. The formula of beryllium oxide is provided as an example.
- Name all the compounds whose formulae you have written.
Table 2.2.3: Some compounds of alkali earth metals

Answers to Questions 2.2.3
Summary about alkali earth metals
- Alkali earth metals have a shiny metallic lusture (appearance).
- Atomic and ionic radii increase down the group because of increasing number of energy levels.
- Hardness, melting point and boiling point decrease down the group because atoms become more loosely held together down the group.
- React by losing two electrons; so they form cations (Be2+, Mg2+, Ca2+).
- Ionization energy decreases down the group because, with increasing energy levels, the outermost electrons are farther from the nucleus, loosely held, and require less energy to knock off.
- Reactivity increases down the group because, with increasing energy levels, the outermost electrons are farther from the nucleus, loosely held, and readily knocked off.
- Alkali earth metals react fairly vigorously with dilute acids, oxygen, chlorine, steam water and many other non-metallic elements.
