CHEMISTRY FORM 2

- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table

- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts

- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Chemical Families and Patterns in Properties: Halogens (Group VII elements)
2.0 Chemical Families and Patterns in Properties
2.3 Halogens (Group VII elements)
Halogen (Greek) means salt producer. The most familiar salt is sodium chloride. Chlorine is therefore considered as a salt producer, and this extends to all the other elements in Group VII where it belongs (fluorine, bromine, iodine e.t.c). They produce salt by combining directly with metals. Figure 2.3.1 shows the appearance of some common halogens.

Figure 2.3.1: Appearance of halogens
2.3.1 Physical properties of halogens
Questions 2.3.1(a)
Study the pictures in Figure 2.3.1, then answer the questions that follow (Q1 & 2).
- Describe the appearance of the following elements in terms of color and physical state.
- Fluorine
- Chlorine
- Bromine
- Iodine
- What is the general trend (change) in physical state as we move from the lightest member (fluorine) to the heavier members such as iodine?
- Study the atomic structures presented in Figure 2.3.2 then answer the questions that follow.

Figure 2.3.2: Atomic structures of halogens
How would you expect melting point (or boiling point) to change as we move from fluorine to iodine? - Halogens have similar properties but are not the same. What feature of atomic structure do all halogens have in common?
Answers to Questions 2.3.1(a)
Halogens are a near-perfect example of smooth trend (change or gradation) in physical properties. We can see from Figure 2.3.1 that the first two members (F2 and Cl2) are gases. The third member (Br2) is a liquid (fuming), and the fourth (I2) is a solid which easily vaporizes, and represents a smooth variation from gas to solid.
From this trend, we can confidently predict that the fifth member is a harder solid than iodine, and that fluorine has a lower boiling point than chlorine. This trend is the reverse of what happens in Groups I and II, where melting points decrease down the groups (Table 2.3).
Table 2.3: Atomic radii and melting points of halogens
| Element | Atomic radius (nm) | Melting point (oC) |
| Flourine | 0.064 | -238 |
| Chlorine | 0.099 | -101 |
| Bromine | 0.114 | -7 |
2.3.2 Chemical properties of halogens
Observe the demonstration of chemical properties of halogens; then answer the following questions (Chlorine is used to represent the other halogens).
(courtesy Youtube-Some Chemical Properties of Chlorine by YESMARK TUTORS)
(courtesy Youtube-BLEACHING ACTION OF CHLORINE GAS by barno donald)
Questions 2.3.1(b)
- What is the effect of chlorine on wet litmus paper?
- What happens when chlorine dissolves in water?
- What is the effect of gaseous halogens on a burning and glowing splint?
- How do halogens smell? (Find out from other sources.)
Answers to Questions 2.3.1(b)
Chlorine reacts with water to form two acids, namely: hydrochloric acid (HCl) and chloric (I) acid (HOCl). Hydrochloric acid turns blue litmus red then chloric (I) acid bleaches it to white immediately. This explains the bleaching action of chlorine on wet litmus paper and other dyes.
The old foolscaps used in the pencasts (Sections 1.4 to 1.9) have turned brown because they were not bleached with chlorine (More in Volume 3).
Solutions of chlorine and bromine in water are called chlorine water and bromine water respectively (Figure 2.3.3).

Figure 2.3.3: (a) Chlorine water (b) Bromine water
When chlorine water is exposed to sunlight, chloric (I) acid readily breaks down (decomposes) to form hydrochloric acid and oxygen gas.

Chlorine water that has been exposed to sunlight therefore turns blue litmus red rather than bleach it.
Preparation of chlorine gas
Chlorine can be prepared by reacting concentrated hydrochloric acid with potassium manganate (VII) crystals or manganese (IV) oxide. Water is used to absorb the fumes of hydrogen chloride gas from hydrochloric acid, while concentrated sulphuric acid is used to dry the gas (Figure 2.3.4).
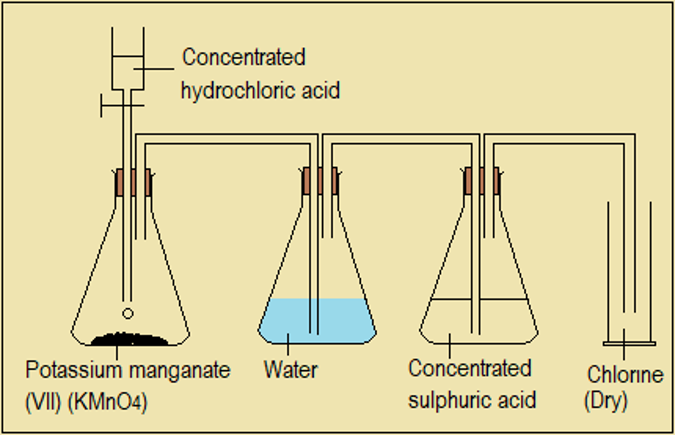
Figure 2.3.4: Laboratory preparation of chlorine gas
NB: Heating is required to speed up the reaction if manganese (IV) oxide is used instead of potassium manganate (VII).
Caution: Halogens are toxic and should only be prepared and used in a functioning fume chamber.
Reaction with metals
We recall the reactions of chlorine with alkali metals and alkali earth metals in Sections 2.1 and 2.3 respectively. The other halogens react in a similar way.
Questions 2.3.1(c)
Write equations for the reactions between
- Magnesium and fluorine
- Calcium and chlorine
- Sodium and bromine
- Potassium and iodine
- Iron and chlorine
- Iron and bromine
Answers to Questions 2.3.1(c)
Halogens combine directly with a wide range of other metals outside Groups I and II, and non-metals. Unlike in metals (Groups I and II), chemical reactivity of halogens decreases down the group from fluorine to iodine and further to astatine. Why?
.Well, halogens lack only one electron to complete their energy levels; so they react by gaining electrons. Gain of electrons occurs more readily in atoms with small atomic radius because their outer energy level to which electron is added is close to the positive nucleus; so the electron is strongly attracted. We say they have a high electron affinity. Fluorine is therefore the most reactive halogen, followed by chlorine.
Questions 2.3.1(d)
Draw a set-up you would use to prepare dry chlorine gas and react it with hot iron powder held in a combustion tube. Remember, the product (iron (III) chloride) sublimes and deposits outside the combustion tube. Label the diagram fully.
Answers to Questions 2.3.1(d)
Summary about halogens
- The first halogens (fluorine and chlorine) are gases, with a gradual change to liquids and solids down the group.
- Atomic and ionic radii increase down the group because of increasing number of energy levels.
- Hardness, melting point and boiling point increase down the group.
- They react by gaining one electron; so they form anions (F-, Cl-, Br-, I-).
- Electron affinity decreases down the group. This is because, with increasing energy levels, nuclear attraction on foreign electrons becomes weaker , making it difficult to gain electrons.
- Reactivity decreases down the group. This is because, with increasing energy levels, the outer energy levers are farther from the nucleus, making it difficult to gain electrons.
- Halogens react with water to form weak acids, and cause bleaching. Also, they react fairly vigorously with metals and some non-metals.
