CHEMISTRY FORM 1





- 1.1 What is matter?
- 1.2 What is Chemistry?
- 1.3 What does matter consist of?
- 1.4 Are the particles in matter stationary?
- 1.5 Arrangement, distance, and attraction between particles
- 1.6 Properties of matter (volume, shape and compression)
- 1.7 Conductors and non-conductors
- 1.8 Sources of heat
- 1.9 Bunsen burner
- 1.10 Role of Chemistry in society

- 2.1 Pure substances
- 2.2 Mixtures
- 2.3 Separation of Mixtures
- 2.4 Separation of solid-solid mixture
- 2.5 Separation of insoluble solid-liquid mixture
- 2.6 Separation of soluble solid-liquid mixture (solution)
- 2.7 Separation of immiscible liquid-liquid mixture
- 2.8 Separation of miscible liquid-liquid mixtures (solution)
- 2.9 Separation of a liquid-gas mixture
- 2.10 Selecting and using appropriate methods of separating mixtures
- 2.11 Kinetic theory of matter
- 2.12 Classification by physical states
- 2.13 Effect of heat on physical states
- 2.14 Effect of impurities on melting and boiling points
- 2.15 Permanent and non-permanent changes
- 2.16 Definitions, chemical symbols and equations
- 3.1 Simple acid-base indicators
- 3.2 Universal indicators and pH scale
- 3.3 Reactions of acids with metals
- 3.4 Reactions of acids with carbonates and hydrogen-carbonates
- 3.5 Reactions of acids with bases
- 3.6 Effects of acids on substances
- 3.7 Applications of acids and bases

- 4.1 Composition of Air
- 4.2 Fractional distillation of liquid air
- 4.3 Rusting
- 4.4 Oxygen
- 4.5 Burning of substances in air
- 4.6 Atmospheric pollution

- 5.1 Candle wax and water
- 5.2 Reactions of metals with liquid water
- 5.3 Reaction of metals with steam
- 5.4 Preparation of hydrogen gas

Air and Combustion: Composition of Air
4.0 Air and Combustion 
4.1 Composition of Air
Air is the mixture of gases surrounding the earth. Air supports lives of animals and plants and supports the combustion of fuels that provide the much needed energy for cooking and industry. It consists of
Nitrogen
Oxygen
Carbon (IV) oxide
Noble (rare) gases
What is the percentage of air that supports combustion?
Materials and substances required
- Measuring cylinder, gas jar or boiling tube
- Trough
- Water, candle, match box and stick
- Metre rule

Caution: Sodium hydroxide is corrosive. Wear gloves or use fresh water.
Watch the demonstration, percentage of air that supports combustion,
Questions 4.1(a)
- Write the procedure for this experiment.
- What is the function of sodium hydroxide solution?
- Describe what happens when the gas jar is inverted to cover the burning candle.
- The height AB of the gas jar was initially occupied by air. How can we proceed to find out the percentage of air used up in the burning of the candle?
- In one such experiment, AB was 20 cm and the height finally occupied by air was 16 cm. Use this information to determine the percentage of air that takes part in burning (active air).
- What is the name of the active gas in air?
- The products of this reaction are water and carbon (IV) oxide. Representing the candle as Wax, write a word equation for the burning of a candle in air.
Answers to Questions 4.1(a)
The same set-up can be used with white phosphorus or wet steel wool instead of the burning candle. But the process involving steel wool is slow and might take 2-3 days. See the set-ups that follow.

Open the video below, reaction of phosphorus and steel wool with air, and take observations.


Questions 4.1(b)
- Describe what happens when
- phosphorus reacts with cold air
- moist steel wool (wet iron) reacts with air
- Write an equation for the reaction between
- phosphorus and oxygen of the air
- steel wool (mainly iron) and oxygen of the air
Answers to Questions 4.1(b)
There are many other possible arrangements which can be used to obtain the percentage of oxygen in air. One of them involves burning copper metal in air.
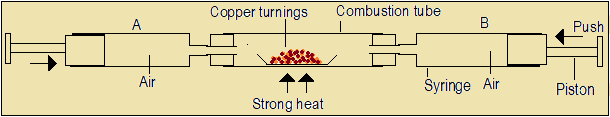
In this arrangement, the total volume (V1) of air in both syringes A and B is noted. Copper turnings are then heated strongly, while pushing the pistons in and out till no further change occurs. The set-up is allowed to cool then the volume (V2) of air remaining is read.
Questions 4.1(c) 
- Why should the pistons be pushed in and out during heating?
- Copper turnings change from red-brown to black during the process. Name the black substance.
- Complete the following equation that would be used to calculate the percentage of air used up.
Percentage of air used = [(----)/---)]x100
- Explain why the percentage obtained may not be accurate.
- Write a word equation for the reaction in this experiment.
Answers to Questions 4.1(c) 
