CHEMISTRY FORM 1





- 1.1 What is matter?
- 1.2 What is Chemistry?
- 1.3 What does matter consist of?
- 1.4 Are the particles in matter stationary?
- 1.5 Arrangement, distance, and attraction between particles
- 1.6 Properties of matter (volume, shape and compression)
- 1.7 Conductors and non-conductors
- 1.8 Sources of heat
- 1.9 Bunsen burner
- 1.10 Role of Chemistry in society
- 2.1 Pure substances
- 2.2 Mixtures
- 2.3 Separation of Mixtures
- 2.4 Separation of solid-solid mixture
- 2.5 Separation of insoluble solid-liquid mixture
- 2.6 Separation of soluble solid-liquid mixture (solution)
- 2.7 Separation of immiscible liquid-liquid mixture
- 2.8 Separation of miscible liquid-liquid mixtures (solution)
- 2.9 Separation of a liquid-gas mixture
- 2.10 Selecting and using appropriate methods of separating mixtures
- 2.11 Kinetic theory of matter
- 2.12 Classification by physical states
- 2.13 Effect of heat on physical states
- 2.14 Effect of impurities on melting and boiling points
- 2.15 Permanent and non-permanent changes
- 2.16 Definitions, chemical symbols and equations
- 3.1 Simple acid-base indicators
- 3.2 Universal indicators and pH scale
- 3.3 Reactions of acids with metals
- 3.4 Reactions of acids with carbonates and hydrogen-carbonates
- 3.5 Reactions of acids with bases
- 3.6 Effects of acids on substances
- 3.7 Applications of acids and bases

- 4.1 Composition of Air
- 4.2 Fractional distillation of liquid air
- 4.3 Rusting
- 4.4 Oxygen
- 4.5 Burning of substances in air
- 4.6 Atmospheric pollution

- 5.1 Candle wax and water
- 5.2 Reactions of metals with liquid water
- 5.3 Reaction of metals with steam
- 5.4 Preparation of hydrogen gas

Air and Combustion: Rusting
4.0 Air and Combustion 
4.3 Rusting
Most metals naturally get destroyed or change colour (tarnish) due to reaction with substances in the environment, a process called corrosion. Copper and other ornaments, corrode and slowly lose their attractiveness (lusture). Corrosion of iron metal and its alloys produces rust, a red-brown loose solid.
What are the conditions necessary for rusting?
Materials and substance required
- Steel wool or iron nails
- Test tubes, some with fitting corks
- Water and oil, e.g. kerosene
- Source of heat (Bunsen burner)
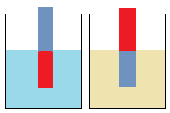
Since rusting occurs everywhere, we suspect that it is caused by conditions which are also available everywhere-especially air or water (or water vapour/moisture). The following set-up is used to find out whether it is air or water, or both.

Anhydrous calcium chloride is a drying agent. Let the set-up stand for about one day.
Questions 4.3(a) 
- Which of the conditions, air and water (or water vapour), are available in
- A -----
- B -----
- C -----
- D -----
- Explain why
- water in B was boiled
- anhydrous calcium chloride is used in C.
- What is the main difference between A and C?
Answers to Questions 4.3(a)
Questions 4.3(b)
- Describe the observations.
- Which conditions are necessary for rusting to occur?
- How does the amount of air available affect rusting?
- What would you do to prevent rusting of iron nails?
- Explain the observations that would be made in the following set-up after 1-2 days. The part of the iron nail above water is dry at the time of setting up the experiment.
- Write a word equation for the formation of rust.

Answers to Questions 4.3(b)
For a fresh iron nail partially dipped in water, more rusting occurs on the exposed part, if maintained wet, than the immersed part. But if kept dry, much more rusting occurs under water than above it.
Questions 4.3(c)
- How can you maintain the exposed part of an iron nail wet?
- While finding out the conditions necessary for rusting, our experiments are usually about air and water. Why do we suspect air or water to be the cause of rusting?
Answers to Questions 4.3(c)
Rusting occurs faster in salty conditions than in fresh water. The salty humid conditions at the coastal areas therefore speed up rusting.

Rusted iron sheets

Rusted steel wool
Question 4.3(d)
From the pictures presented here and in the table of Common Chemistry Laboratory Chemicals, identify two substances present in a rusted iron sheet.
Answer to Question 4.3(d)
Rust is porous. Therefore, it allows in water and air to penetrate and cause damage underneath, finally destroying the whole metal. The metal is converted into a loose powder. Iron sheets are normally protected by coating them with zinc and are then called galvanized sheets.
Protection from rusting
- Alloying (e.g. with carbon to produce rust-resistant steel used on railway lines)
- Painting (e.g. of steel windows and bodies of motor vehicles)
- Coating (plating) with other metals (e.g. with chromium or silver for cutlery)
- Oiling and greasing (e.g. of nails and farm tools)
- Galvanizing; that is, coating iron sheets with zinc—being more resistant to corrosion
