CHEMISTRY FORM 1





- 1.1 What is matter?
- 1.2 What is Chemistry?
- 1.3 What does matter consist of?
- 1.4 Are the particles in matter stationary?
- 1.5 Arrangement, distance, and attraction between particles
- 1.6 Properties of matter (volume, shape and compression)
- 1.7 Conductors and non-conductors
- 1.8 Sources of heat
- 1.9 Bunsen burner
- 1.10 Role of Chemistry in society
- 2.1 Pure substances
- 2.2 Mixtures
- 2.3 Separation of Mixtures
- 2.4 Separation of solid-solid mixture
- 2.5 Separation of insoluble solid-liquid mixture
- 2.6 Separation of soluble solid-liquid mixture (solution)
- 2.7 Separation of immiscible liquid-liquid mixture
- 2.8 Separation of miscible liquid-liquid mixtures (solution)
- 2.9 Separation of liquid-gas mixture
- 2.10 Selecting and using appropriate methods of separating mixtures
- 2.11 Kinetic theory of matter
- 2.12 Classification by physical states
- 2.13 Effect of heat on physical states
- 2.14 Effect of impurities on melting and boiling points
- 2.15 Permanent and non-permanent changes
- 2.16 Definitions, chemical symbols and equations
- 3.1 Simple acid-base indicators
- 3.2 Universal indicators and pH scale
- 3.3 Reactions of acids with metals
- 3.4 Reactions of acids with carbonates and hydrogen-carbonates
- 3.5 Reactions of acids with bases
- 3.6 Effects of acids on substances
- 3.7 Applications of acids and bases

- 4.1 Composition of Air
- 4.2 Fractional distillation of liquid air
- 4.3 Rusting
- 4.4 Oxygen
- 4.5 Burning of substances in air
- 4.6 Atmospheric pollution

- 5.1 Candle wax and water
- 5.2 Reactions of metals with liquid water
- 5.3 Reaction of metals with steam
- 5.4 Preparation of hydrogen gas

Introduction to Chemistry: Are the particles in matter stationary?
1.0 Introduction to Chemistry
1.4 Are the particles in matter stationary?
To find out if the particles in matter are stationary or moving
Materials and substances required
- Smoke cell or dark and dusty room
- A 3-cell torch or laser pointer as a source of a powerful beam of light
- Clean water, and small particles such as of dry processed tea leaves

Open the video, dust particles suspended in air, and take observations keenly.
Questions 1.4(a)
From your observations
- Are the suspended particles moving or still?
- If moving
- in which direction are they doing so: down, up, across, diagonal or all directions?
- does the average speed increase, decrease or remain unchanged as time goes on?
- Dust particles, such as those on the desk, cannot move on their own. What causes (pushes) them to move in air or water?
- In what manner then do the particles of air or water move?
- Do you think that the movement will stop after some time? Explain.
Answers to Questions 1.4(a)
In conclusion, particles of gases and liquids move continuously in all directions. Movement in all directions is called random motion, or Brownian motion.
In solids (not demonstrated), particles move to-and-fro. That is, they vibrate at fixed positions. Overall, particles in matter are in continuous motion. This is referred to as the kinetic theory of matter.
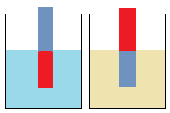
What is the effect of temperature (degree of hotness or coldness) on Brownian motion?
Observe keenly the behavior of dust particles suspended in cold water,
and again when suspended in hot water.
Questions 1.4(b)
- How does an increase in temperature affect the movement of particles in water?
- In which of the following substances is movement of particles fastest? Give a reason for your answer. hot water, cold water, oxygen gas
- How does the movement of particles in solids differ from the movement in liquids and gases?
- In what ways is the movement of particles in solids similar to movement in liquids and gases?
- How does Brownian motion differ from wind motion?
Answers to Questions 1.4(b)
At home
Dust particles move in a random (zigzag, haphazard, jittery) manner as viewed in the headlights of a car at night or in a direct sunbeam passing through a hole in the roof or wall.

Beam of light passing through a roof
Suspended dust particles in water held in a drinking glass move continuously. Vibrations can be felt in cooking vessels (e.g. sufuria) as they are heated. All these observations indicate that particles of matter are in motion.
