CHEMISTRY FORM 1





- 1.1 What is matter?
- 1.2 What is Chemistry?
- 1.3 What does matter consist of?
- 1.4 Are the particles in matter stationary?
- 1.5 Arrangement, distance, and attraction between particles
- 1.6 Properties of matter (volume, shape and compression)
- 1.7 Conductors and non-conductors
- 1.8 Sources of heat
- 1.9 Bunsen burner
- 1.10 Role of Chemistry in society

- 2.1 Pure substances
- 2.2 Mixtures
- 2.3 Separation of Mixtures
- 2.4 Separation of solid-solid mixture
- 2.5 Separation of insoluble solid-liquid mixture
- 2.6 Separation of soluble solid-liquid mixture (solution)
- 2.7 Separation of immiscible liquid-liquid mixture
- 2.8 Separation of miscible liquid-liquid mixtures (solution)
- 2.9 Separation of liquid-gas mixture
- 2.10 Selecting and using appropriate methods of separating mixtures
- 2.11 Kinetic theory of matter
- 2.12 Classification by physical states
- 2.13 Effect of heat on physical states
- 2.14 Effect of impurities on melting and boiling points
- 2.15 Permanent and non-permanent changes
- 2.16 Definitions, chemical symbols and equations
- 3.1 Simple acid-base indicators
- 3.2 Universal indicators and pH scale
- 3.3 Reactions of acids with metals
- 3.4 Reactions of acids with carbonates and hydrogen-carbonates
- 3.5 Reactions of acids with bases
- 3.6 Effects of acids on substances
- 3.7 Applications of acids and bases

- 4.1 Composition of Air
- 4.2 Fractional distillation of liquid air
- 4.3 Rusting
- 4.4 Oxygen
- 4.5 Burning of substances in air
- 4.6 Atmospheric pollution

- 5.1 Candle wax and water
- 5.2 Reactions of metals with liquid water
- 5.3 Reaction of metals with steam
- 5.4 Preparation of hydrogen gas

Introduction to Chemistry: Properties of matter (volume, shape and compression)
1.0 Introduction to Chemistry
1.6 Properties of matter (volume, shape and compression)
Demonstrations
Solid
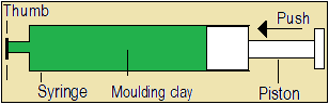
A1 Play the video about volume and shape of solids, below.
- Read the volume of moulding clay in the syringe.
- Observe any change in the volume of moulding clay as the piston is pushed inwards.
- Observe any change in the shape of moulding clay as the piston is pulled outwards while the syringe is horizontal.
Questions 1.6(a)i
NB: Pushing does not mean motion. We may push something but it fails to move.
A2 From the observations in A1:
- Does the volume or size of moulding clay become smaller as the piston is pushed inwards?
- Is moulding clay (a solid) compressible?
- Is the shape of moulding clay affected by withdrawing the piston?
Liquid
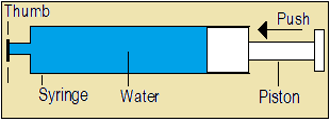
B1 Click on the link volume and shape of liquids.
- Read the volume of water sucked into the syringe.
- Observe any change in the volume of water as the piston is pushed inwards.
- Observe any change in the shape of water as the piston is pulled outwards while the syringe is horizontal, slanted and vertical.
Questions 1.6(a)ii
B2 From the observations in B1:
- Does the volume of water reduce on pushing the piston inwards?
- Is water compressible?
- Does the shape of water change as the syringe is tilted?
Gas

C1 Click the link volume and shape of gases.
- Note the volume of air trapped in the syringe.
- Observe any change in the volume of air as the piston is pushed inwards then released.
- Think of all possible shapes of a container, not holding any solid or liquid. Naturally, air fills it up.
Questions 1.6(a)iii
C2 From the observations in C1:
- Does the volume of air reduce on pushing the piston inwards?
- Is air compressible?
- How does the shape of air relate to the shape of the container holding it?
Answers to Questions 1.6(a) 
Questions 1.6(b) (Conclusions)
- What conclusion can we make about volume and shape of solids?
- Make a conclusion about volume and shape of liquids.
- Write a conclusion about volume and shape of gases. NB: The conclusions should indicate whether volume and shape are fixed (definite) or not, and whether they take the shape of the container.
- Bonus question
How can we show that compressing or expanding a gas does not change its mass?
Answers to Questions 1.6(b) (Conclusions)
We can easily observe that solids and liquids occupy space, because they are visible. For gases, the springing back of piston shows that they also occupy space. Therefore, as stated earlier, matter occupies space.
At home
Houses, containers and other objects maintain their shapes because they are made of solids, which have definite shapes. Bottled water, milk, juice and soda are priced by volume because, as liquids, they have definite volume.
Cooking gas is priced by mass in kilograms (kg) because its volume can take any value, depending on compression, and therefore not reliable. Air is used in cushions and tyres of cars and motor bikes, because it is compressible, absorbs shock, and therefore comfortable.
NB: The definite shape of solids does not mean regular or unchangeable shape. It means that solids retain their shape, whether regular or not, even when a container is removed. When we mould clay into a ball or toy, for example, it retains that shape till we change it.
