CHEMISTRY FORM 1





- 1.1 What is matter?
- 1.2 What is Chemistry?
- 1.3 What does matter consist of?
- 1.4 Are the particles in matter stationary?
- 1.5 Arrangement, distance, and attraction between particles
- 1.6 Properties of matter (volume, shape and compression)
- 1.7 Conductors and non-conductors
- 1.8 Sources of heat
- 1.9 Bunsen burner
- 1.10 Role of Chemistry in society

- 2.1 Pure substances
- 2.2 Mixtures
- 2.3 Separation of Mixtures
- 2.4 Separation of solid-solid mixture
- 2.5 Separation of insoluble solid-liquid mixture
- 2.6 Separation of soluble solid-liquid mixture (solution)
- 2.7 Separation of immiscible liquid-liquid mixture
- 2.8 Separation of miscible liquid-liquid mixtures (solution)
- 2.9 Separation of liquid-gas mixture
- 2.10 Selecting and using appropriate methods of separating mixtures
- 2.11 Kinetic theory of matter
- 2.12 Classification by physical states
- 2.13 Effect of heat on physical states
- 2.14 Effect of impurities on melting and boiling points
- 2.15 Permanent and non-permanent changes
- 2.16 Definitions, chemical symbols and equations
- 3.1 Simple acid-base indicators
- 3.2 Universal indicators and pH scale
- 3.3 Reactions of acids with metals
- 3.4 Reactions of acids with carbonates and hydrogen-carbonates
- 3.5 Reactions of acids with bases
- 3.6 Effects of acids on substances
- 3.7 Applications of acids and bases

- 4.1 Composition of Air
- 4.2 Fractional distillation of liquid air
- 4.3 Rusting
- 4.4 Oxygen
- 4.5 Burning of substances in air
- 4.6 Atmospheric pollution

- 5.1 Candle wax and water
- 5.2 Reactions of metals with liquid water
- 5.3 Reaction of metals with steam
- 5.4 Preparation of hydrogen gas

Simple Classification of Substances and Separation of Mixtures: Seperation of solid solid of Mixtures
2.0 Simple Classification of Substances and Separation of Mixtures 
2.4 Separation of solid-solid mixture
How can we separate a mixture of iron filings and sand?
Open the video, separation of iron filings and sand.
Questions 2.4(a)
- What difference between sand and iron does this separation rely on?
- Suggest a name for this method of separation.
- Which other substances behave in the same manner as iron?
- Name two other binary mixtures, with iron filings as a component, which can be separated using this method.
Answers to Questions 2.4(a)
In industry
Magnetic picking is used to sort scrap metals from non-metals for recycling, and in loading.
Open the video below.
In hospital
In hospitals, magnetic picking can be used to remove magnetic specks that accidentally get into the eye.At home
Beans, peas, groundnuts, maize, rice or flour mixed with sand or small stones, gains, and chaff are common examples of solid-solid mixtures.
Question: Find out the traditional methods of separating such mixtures.
How can we separate sand and iodine?
Materials and substances required
- A mixture of sand and iodine
- Source of heat (Bunsen burner and laboratory/cooking gas)
- Glass beaker, water, watch glass
- Tripod stand and wire gauze

Sublimation
Open the video below, separation of iodine from sand,
Questions 2.4(b) 
- What difference between sand and iodine does this separation rely on?
- Suggest a name for this method of separation.
- Which other substances behave in the same manner as iodine?
- Outline the steps used in this method of separation.
- What is the use of water or ice held in the watch glass?
- Like sand, sodium chloride does not sublime. Name five (5) other pairs of loose solids that can be separated by sublimation (leave out carbon (IV) oxide).
Open the video separation of sodium chloride and iodine.
Caution: In an actual experiment, take care not to inhale the dense purple iodine vapour. This can be done by using a fume chamber, heating slowly and using chilled water or ice to cool the vapour.
Answers to Questions 2.4(b)
NB: In sublimation, the non-volatile substance should not melt or react with air during heating. Cane sugar, which readily melts, cannot be separated from iodine by sublimation.
In life
Sublimation, like melting, occurs with absorption of heat and therefore has a cooling effect. Ice vendors therefore use carbon (IV) oxide (dry ice), which sublimes, to keep ice cream frozen. It is a more effective coolant than ice water (wet ice).
How can we separate a mixture of sand and sodium chloride?
Materials and substances required
- Beakers, conical flask, filter paper, funnel, glass rod (or stirrer)
- Source of heat (Bunsen burner), tripod stand, wire gauze,
- Water
- The mixture of sodium chloride and sand

Open the link, separation of sand and sodium chloride.
Observe how the filter paper is folded and used, and all other steps in the method.
Question 2.4(c) 
- What difference between sand and sodium chloride does this separation rely on?
- Water is added to dissolve one component of the mixture. What is the general name of liquids that dissolve other substances?
- Suggest a name for this method of separation.
- What is the general name of the liquid component that passes through the filter paper?
- Outline the procedure for this method of separation.
- Name at least four (4) other substances which share the same property (solubility) shown by sodium chloride.
- Sulphur and charcoal, are similar to sand in terms of not being soluble. Name any two other pairs (binary mixtures) that can be separated using this method.
-
- What is observed on the filter paper at the end of the process?
- What do we call the substance that remains on the filter paper?
- Where do we use this method in real life?
Answers to Questions 2.4(c)
At home
In traditional societies, this method (solvent extraction) has been used to extract soluble components of wood ash used in quick softening of fresh vegetables. This preserves the natural green colour of vegetable as it cooks.
Questions 2.4(d) No 1
For each of the following mixtures, identify the best method of separation.
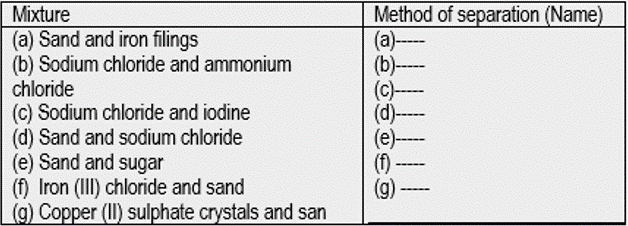
Answers to Questions 2.4(d) No. 1 
NB: For some solid mixtures such as sugar and sodium chloride, both components dissolve in water, heating destroys one of them (sugar), and both are non-magnetic. Such mixtures required more advanced methods to separate.
Questions 2.4(d) No 2 
Describe how you would separate each of the following solid-solid mixtures.
- Sand and iodine
- Sulphur and iron filings
- Copper (II) sulphate crystals and sand
Answers to Questions 2.4(d) No. 2
Questions 2.4(d) No 3 
A mixture consists of small particles of solids X and Y. When heated, solid X easily melts into a thin liquid but does not dissolve Y. X and Y are non-magnetic and do not sublime. Neither are they destroyed by heat. Explain how you would obtain pure solid X and Y from the mixture.
Answers to Questions 2.4(d) No. 3 
