CHEMISTRY LEVEL 3
- 1.1 Boyle's Law
- 1.2 Charles'law
- 1.3 Combined gas law
- 1.4 Standard conditions
- 1.5 Diffusion and Graham's law
- 2.1 Relative Mass
- 2.2 Atoms, Molecules and Moles
- 2.3 Compounds and the mole
- 2.4 Empirical and Molecular formula
- 2.5 Concentration of a solution
- 2.6 Molar solutions
- 2.7 Preparation of molar solutions
- 2.8 Dilution of a solution
- 2.9 Stoichiometry of chemical reactions
- 2.10 Volumetric analysis
- 2.11 Titration
- 2.12 Redox titration
- 2.13 Atomicity and molar gas volume
- 2.14 Combining volumes of gases
- 3.1 Alkanes
- 3.1.1 Formulae of alkanes
- 3.1.2 Cracking of alkanes
- 3.1.3 Nomenclature (systematic naming) of alkanes
- 3.1.4 Isomerism in alkanes
- 3.1.5 Laboratory preparation of alkanes
- 3.1.6 Physical properties of alkanes
- 3.1.7 Chemical properties of alkanes
- 3.1.8 Uses of alkanes
- 3.2 Alkenes
- 3.2.1 Nomenclature of alkenes
- 3.2.2 Isomerism in alkenes
- 3.2.3 Laboratory preparation of ethene
- 3.2.4 Physical properties of alkenes
- 3.2.5 Chemical properties of alkenes
- 3.2.6 Test for alkenes
- 3.2.7 Uses of alkenes
- 3.3 Alkynes
- 3.3.1 Nomenclature of alkynes
- 3.3.2 Isomerism in alkynes
- 3.3.3 Laboratory preparation of ethyne
- 3.3.4 Physical properties of alkynes
- 3.3.5 Chemical properties of alkynes
- 3.3.6 Test for alkynes
- 3.3.7 Uses of alkynes
- 3.4 Recommended practice of topic summary
- 4.1 Extraction of nitrogen from air
- 4.2.1 Laboratory preparation of nitrogen gas from the air
- 4.2.2 Laboratory preparation of nitrogen gas from ammonium nitrite ((NH4NO2))
- 4.2.3 Uses of nitrogen
- 4.3 Oxides of nitrogen
- 4.3.1 Nitrogen (I) oxide
- 4.3.2 Nitrogen (II) oxide
- 4.3.3 Nitrogen (IV) oxide
- 4.4.1 Laboratory preparation of ammonia
- 4.4.2 Solubility of ammonia in water
- 4.4.3 Reactions of aqueous ammonia (ammonia solution)
- 4.4.4 Reactions of ammonia gas
- 4.4.5 Industrial manufacture of ammonia: The Haber Process
- 4.4.6 Uses of ammonia
- 4.4.7 Nitrogenous fertilizers
- 4.5.1 Laboratory preparation of nitric (V) acid
- 4.5.2 Industrial manufacture of nitric (V) acid
- 4.5.3 Reactions of dilute nitric (V) acid
- 4.5.4 Reactions of concentrated nitric (V) acid
- 4.5.5 Uses of nitric (V) acid
- 4.6.1 Action of heat on nitrates
- 4.6.2 Test for nitrates (nitrate ions, NO3-)
- 4.6.3 Air pollution by nitrogen compounds
- 4.7 Summary on nitrogen and its compounds
- 5.0 Sulphur and its Compounds
- 5.1.1 Extraction of sulphur
- 5.1.2 Allotropes of sulphur
- 5.1.3 Physical properties of sulphur
- 5.1.4 Chemical properties of sulphur
- 5.2.1 Preparation of sulphur (IV) oxide
- 5.2.2 Physical properties of sulphur (IV) oxide
- 5.2.3 Chemical properties of sulphur (IV) oxide
- 5.2.4 Reducing action of sulphur (IV) oxide
- 5.2.5 Oxidization of SO2 to SO3
- 5.2.6 Oxidizing action of sulphur (IV) oxide
- 5.2.7 Test for sulphite (SO32-) and sulphate (SO42-) ions
- 5.2.8 Uses of sulphur (IV) oxide
- 5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
- 5.3.1 Physical properties of concentrated sulphuric (VI) acid
- 5.3.2 Chemical properties of concentrated sulphuric (VI) acid
- 5.3.3 Reactions of dilute sulphuric (VI) acid
- 5.4 Hydrogen sulphide
- 5.4.1 Chemical properties of hydrogen sulphide
- 5.4.2 Air pollution by compounds of sulphur
- 5.5 Summary on sulphur and its compounds
- 6.1 Occurrence of chlorine
- 6.2 Laboratory preparation of chlorine
- 6.3 Physical properties of chlorine
- 6.4 Chemical properties of chlorine
- 6.5 Oxidizing properties of chlorine
- 6.6 Reaction of chlorine with alkaline solutions
- 6.7 Test for chloride ions
- 6.8 Uses of chlorine and its compounds
- 6.9 Preparation of hydrogen chloride gas
- 6.10 Physical properties of hydrogen chloride
- 6.11 Chemical properties of hydrogen chloride
- 6.12 Industrial manufacture of hydrochloric acid
- 6.13 Uses of hydrochloric acid


Sulphur and its Compounds: Chemical properties of sulphur
5.0 Sulphur and its Compounds
5.1.4: Chemical properties of sulphur
Burning in oxygen (and air)
The following video clip and photographs show the burning of sulphur in air. The whiteness seen on the deflagrating spoon is an effect of the camera lens, not the actual colour.
(courtesy Youtube-Combustion of sulphur in oxygen by Chemistry Channel)

Figure 5.1.4(a): Burning of sulphur in oxygen
https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcQ5mhH8HkMPzZvfpvmkaVi1rEet31xNDnfeeg&usqp=CAU
Questions 5.1.4(a)
- Outline a three-step procedure you would use to demonstrate the burning of sulphur in air, using a gas jar full of oxygen gas.
- State the observations made during the burning.
- Sulphur shows a valency of 4 in its reaction with oxygen. Name the product and state its formula.
- Write a chemical equation for the burning of sulphur in oxygen (or air).
- Determine the volume of oxygen gas at room temperature and pressure required to react completely with 16 g of sulphur (S = 32; molar gas volume at r.t.p = 24 dm3).
Answers to Questions 5.1.4(a) 
Direct combination of sulphur with iron
The following photographs show what happens when a mixture of iron dust and sulphur powder is strongly heated in a Bunsen flame.

Figure 5.1.4(b): Reaction between sulphur and iron
Questions 5.1.4(b)
- State the observations made.
- Iron shows a valency of 2 in its direct combination with sulphur. Name the product and state its formula.
- Write an equation for the reaction between sulphur and iron.
- Explain the observation made on the cooler parts of the test tube.
Answers to Questions 5.1.4(b) 
Direct combination with copper
The following photographs show what happens when a mixture of copper turnings and sulphur powder is strongly heated in a Bunsen flame.

Figure 5.1.4(c): Direct combination of sulphur with copper
Questions 5.1.4(c)
- Describe the observations made.
- Copper and sulphur show a valency of 1 and 2 respectively in their direct combination with each other. Name the product and state its formula.
- Write an equation for the reaction between sulphur and copper.
Answers to Questions 5.1.4(c) 
Direct combination of sulphur with hydrogen
The following photographs show what happens when hydrogen gas is blown over burning sulphur.
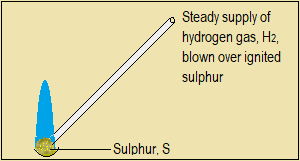
Figure 5.1.4(d): Direct combination of sulphur with hydrogen
Caution: Do not attempt to lower burning sulphur into a gas jar full of hydrogen.
Questions 5.1.4(d)
- Give two reasons why it is not advisable to react sulphur with hydrogen by lowering burning sulphur into a gas jar containing hydrogen.
- Describe the observations made.
- Sulphur shows a valency of 2 in its compound with hydrogen. State the formula of the compound.
- Write an equation for the reaction between sulphur and hydrogen.
Answers to Questions 5.1.4(d) 
