×








CHEMISTRY LEVEL 3
1. GAS LAWS
- 1.1 Boyle's Law
- 1.2 Charles'law
- 1.3 Combined gas law
- 1.4 Standard conditions
- 1.5 Diffusion and Graham's law
2. THE MOLE: Formulae and Chemical Equations
- 2.1 Relative Mass
- 2.2 Atoms, Molecules and Moles
- 2.3 Compounds and the mole
- 2.4 Empirical and Molecular formula
- 2.5 Concentration of a solution
- 2.6 Molar solutions
- 2.7 Preparation of molar solutions
- 2.8 Dilution of a solution
- 2.9 Stoichiometry of chemical reactions
- 2.10 Volumetric analysis
- 2.11 Titration
- 2.12 Redox titration
- 2.13 Atomicity and molar gas volume
- 2.14 Combining volumes of gases
3. ORGANIC CHEMISTRY 1
- 3.1 Alkanes
- 3.1.1 Formulae of alkanes
- 3.1.2 Cracking of alkanes
- 3.1.3 Nomenclature (systematic naming) of alkanes
- 3.1.4 Isomerism in alkanes
- 3.1.5 Laboratory preparation of alkanes
- 3.1.6 Physical properties of alkanes
- 3.1.7 Chemical properties of alkanes
- 3.1.8 Uses of alkanes
- 3.2 Alkenes
- 3.2.1 Nomenclature of alkenes
- 3.2.2 Isomerism in alkenes
- 3.2.3 Laboratory preparation of ethene
- 3.2.4 Physical properties of alkenes
- 3.2.5 Chemical properties of alkenes
- 3.2.6 Test for alkenes
- 3.2.7 Uses of alkenes
- 3.3 Alkynes
- 3.3.1 Nomenclature of alkynes
- 3.3.2 Isomerism in alkynes
- 3.3.3 Laboratory preparation of ethyne
- 3.3.4 Physical properties of alkynes
- 3.3.5 Chemical properties of alkynes
- 3.3.6 Test for alkynes
- 3.3.7 Uses of alkynes
- 3.4 Recommended practice of topic summary
4. NITROGEN AND ITS COMPOUNDS
- 4.1 Extraction of nitrogen from air
- 4.2.1 Laboratory preparation of nitrogen gas from the air
- 4.2.2 Laboratory preparation of nitrogen gas from ammonium nitrite ((NH4NO2))
- 4.2.3 Uses of nitrogen
- 4.3 Oxides of nitrogen
- 4.3.1 Nitrogen (I) oxide
- 4.3.2 Nitrogen (II) oxide
- 4.3.3 Nitrogen (IV) oxide
- 4.4.1 Laboratory preparation of ammonia
- 4.4.2 Solubility of ammonia in water
- 4.4.3 Reactions of aqueous ammonia (ammonia solution)
- 4.4.4 Reactions of ammonia gas
- 4.4.5 Industrial manufacture of ammonia: The Haber Process
- 4.4.6 Uses of ammonia
- 4.4.7 Nitrogenous fertilizers
- 4.5.1 Laboratory preparation of nitric (V) acid
- 4.5.2 Industrial manufacture of nitric (V) acid
- 4.5.3 Reactions of dilute nitric (V) acid
- 4.5.4 Reactions of concentrated nitric (V) acid
- 4.5.5 Uses of nitric (V) acid
- 4.6.1 Action of heat on nitrates
- 4.6.2 Test for nitrates (nitrate ions, NO3-)
- 4.6.3 Air pollution by nitrogen compounds
- 4.7 Summary on nitrogen and its compounds
5. SULPHUR AND ITS COMPOUNDS
- 5.0 Sulphur and its Compounds
- 5.1.1 Extraction of sulphur
- 5.1.2 Allotropes of sulphur
- 5.1.3 Physical properties of sulphur
- 5.1.4 Chemical properties of sulphur
- 5.2.1 Preparation of sulphur (IV) oxide
- 5.2.2 Physical properties of sulphur (IV) oxide
- 5.2.3 Chemical properties of sulphur (IV) oxide
- 5.2.4 Reducing action of sulphur (IV) oxide
- 5.2.5 Oxidization of SO2 to SO3
- 5.2.6 Oxidizing action of sulphur (IV) oxide
- 5.2.7 Test for sulphite (SO32-) and sulphate (SO42-) ions
- 5.2.8 Uses of sulphur (IV) oxide
- 5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
- 5.3.1 Physical properties of concentrated sulphuric (VI) acid
- 5.3.2 Chemical properties of concentrated sulphuric (VI) acid
- 5.3.3 Reactions of dilute sulphuric (VI) acid
- 5.4 Hydrogen sulphide
- 5.4.1 Chemical properties of hydrogen sulphide
- 5.4.2 Air pollution by compounds of sulphur
- 5.5 Summary on sulphur and its compounds
6. CHLORINE AND ITS COMPOUNDS
- 6.1 Occurrence of chlorine
- 6.2 Laboratory preparation of chlorine
- 6.3 Physical properties of chlorine
- 6.4 Chemical properties of chlorine
- 6.5 Oxidizing properties of chlorine
- 6.6 Reaction of chlorine with alkaline solutions
- 6.7 Test for chloride ions
- 6.8 Uses of chlorine and its compounds
- 6.9 Preparation of hydrogen chloride gas
- 6.10 Physical properties of hydrogen chloride
- 6.11 Chemical properties of hydrogen chloride
- 6.12 Industrial manufacture of hydrochloric acid
- 6.13 Uses of hydrochloric acid
7. A guide to chemical tests based on this module

Content developer

Nitrogen and its Compounds: Extraction of nitrogen from air
4.0 Nitrogen and its Compounds

4.1 Extraction of nitrogen from air
Normally, substances used in industrial manufacture are extracted from their most abundant sources. For nitrogen, the abundant source is the atmosphere, where it makes up 78% and weighs over 4 trillion tons. It is therefore extracted by fractional distillation of liquefied air (Figure 4.1), as in the extraction of petroleum fuels from crude oil.
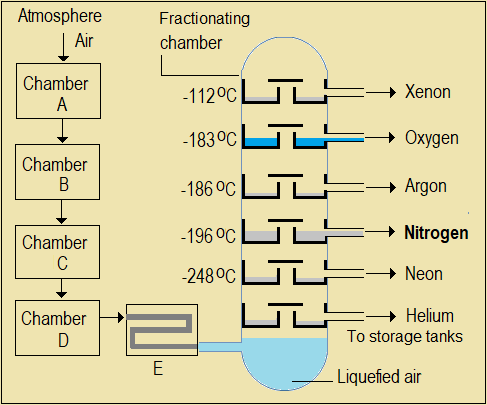
Figure 4.1: Fractional distillation of liquefied air (ANIM)
(courtesy Youtube-Preparation of Nitrous oxide (laughing gas) or Dinitrogen monoxide by Shiva Chemist)
(courtesy Youtube-Chemical properties of Nitrous oxide (laughing gas) by Shiva Chemist)
Questions 4.1
- First, we need a Chamber, A, to set air flowing continuously from the atmosphere into Chamber B and further into the distillation column. Name the device, Chamber A.
- Atmospheric air contains dust, water vapour, and carbon (IV) oxide which must be removed. How can dust be removed? Suggest a name for Chamber B.
- When compressed and cooled a little below room temperature, water vapour readily condenses, while carbon (IV) oxide turns to solid (undergoes deposition) at -78.5 oC on further cooling. (a) What is the function of Chamber C? (b) Name the substances tapped from Chamber C and D respectively. (c) Suggest one use of the by-product obtained from Chamber D.
- Explain why, although sodium hydroxide solution absorbs carbon (IV) oxide gas, it is not suitable for removing the gas in this process.
- Name the process that takes place in Chamber E.
- During distillation, liquid air is allowed to evaporate and distill as fractions. Identify one way in which this process is (a) similar to distillation of crude oil (b) differs from the distillation of crude oil.
- Suggest a reason why air should be liquefied before its components are separated.
- An air distillation plant is considered as an economical and profitable investment. Suggest two reasons for this.
Answers to Questions 4.1 
