×








CHEMISTRY LEVEL 3
1. GAS LAWS
- 1.1 Boyle's Law
- 1.2 Charles'law
- 1.3 Combined gas law
- 1.4 Standard conditions
- 1.5 Diffusion and Graham's law
2. THE MOLE: Formulae and Chemical Equations
- 2.1 Relative Mass
- 2.2 Atoms, Molecules and Moles
- 2.3 Compounds and the mole
- 2.4 Empirical and Molecular formula
- 2.5 Concentration of a solution
- 2.6 Molar solutions
- 2.7 Preparation of molar solutions
- 2.8 Dilution of a solution
- 2.9 Stoichiometry of chemical reactions
- 2.10 Volumetric analysis
- 2.11 Titration
- 2.12 Redox titration
- 2.13 Atomicity and molar gas volume
- 2.14 Combining volumes of gases
3. ORGANIC CHEMISTRY 1
- 3.1 Alkanes
- 3.1.1 Formulae of alkanes
- 3.1.2 Cracking of alkanes
- 3.1.3 Nomenclature (systematic naming) of alkanes
- 3.1.4 Isomerism in alkanes
- 3.1.5 Laboratory preparation of alkanes
- 3.1.6 Physical properties of alkanes
- 3.1.7 Chemical properties of alkanes
- 3.1.8 Uses of alkanes
- 3.2 Alkenes
- 3.2.1 Nomenclature of alkenes
- 3.2.2 Isomerism in alkenes
- 3.2.3 Laboratory preparation of ethene
- 3.2.4 Physical properties of alkenes
- 3.2.5 Chemical properties of alkenes
- 3.2.6 Test for alkenes
- 3.2.7 Uses of alkenes
- 3.3 Alkynes
- 3.3.1 Nomenclature of alkynes
- 3.3.2 Isomerism in alkynes
- 3.3.3 Laboratory preparation of ethyne
- 3.3.4 Physical properties of alkynes
- 3.3.5 Chemical properties of alkynes
- 3.3.6 Test for alkynes
- 3.3.7 Uses of alkynes
- 3.4 Recommended practice of topic summary
4. NITROGEN AND ITS COMPOUNDS
- 4.1 Extraction of nitrogen from air
- 4.2.1 Laboratory preparation of nitrogen gas from the air
- 4.2.2 Laboratory preparation of nitrogen gas from ammonium nitrite ((NH4NO2))
- 4.2.3 Uses of nitrogen
- 4.3 Oxides of nitrogen
- 4.3.1 Nitrogen (I) oxide
- 4.3.2 Nitrogen (II) oxide
- 4.3.3 Nitrogen (IV) oxide
- 4.4.1 Laboratory preparation of ammonia
- 4.4.2 Solubility of ammonia in water
- 4.4.3 Reactions of aqueous ammonia (ammonia solution)
- 4.4.4 Reactions of ammonia gas
- 4.4.5 Industrial manufacture of ammonia: The Haber Process
- 4.4.6 Uses of ammonia
- 4.4.7 Nitrogenous fertilizers
- 4.5.1 Laboratory preparation of nitric (V) acid
- 4.5.2 Industrial manufacture of nitric (V) acid
- 4.5.3 Reactions of dilute nitric (V) acid
- 4.5.4 Reactions of concentrated nitric (V) acid
- 4.5.5 Uses of nitric (V) acid
- 4.6.1 Action of heat on nitrates
- 4.6.2 Test for nitrates (nitrate ions, NO3-)
- 4.6.3 Air pollution by nitrogen compounds
- 4.7 Summary on nitrogen and its compounds
5. SULPHUR AND ITS COMPOUNDS
- 5.0 Sulphur and its Compounds
- 5.1.1 Extraction of sulphur
- 5.1.2 Allotropes of sulphur
- 5.1.3 Physical properties of sulphur
- 5.1.4 Chemical properties of sulphur
- 5.2.1 Preparation of sulphur (IV) oxide
- 5.2.2 Physical properties of sulphur (IV) oxide
- 5.2.3 Chemical properties of sulphur (IV) oxide
- 5.2.4 Reducing action of sulphur (IV) oxide
- 5.2.5 Oxidization of SO2 to SO3
- 5.2.6 Oxidizing action of sulphur (IV) oxide
- 5.2.7 Test for sulphite (SO32-) and sulphate (SO42-) ions
- 5.2.8 Uses of sulphur (IV) oxide
- 5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
- 5.3.1 Physical properties of concentrated sulphuric (VI) acid
- 5.3.2 Chemical properties of concentrated sulphuric (VI) acid
- 5.3.3 Reactions of dilute sulphuric (VI) acid
- 5.4 Hydrogen sulphide
- 5.4.1 Chemical properties of hydrogen sulphide
- 5.4.2 Air pollution by compounds of sulphur
- 5.5 Summary on sulphur and its compounds
6. CHLORINE AND ITS COMPOUNDS
- 6.1 Occurrence of chlorine
- 6.2 Laboratory preparation of chlorine
- 6.3 Physical properties of chlorine
- 6.4 Chemical properties of chlorine
- 6.5 Oxidizing properties of chlorine
- 6.6 Reaction of chlorine with alkaline solutions
- 6.7 Test for chloride ions
- 6.8 Uses of chlorine and its compounds
- 6.9 Preparation of hydrogen chloride gas
- 6.10 Physical properties of hydrogen chloride
- 6.11 Chemical properties of hydrogen chloride
- 6.12 Industrial manufacture of hydrochloric acid
- 6.13 Uses of hydrochloric acid
7. A guide to chemical tests based on this module

Content developer

Sulphur and its Compounds:Large scale (industrial) manufacture of sulphuric (VI) acid
5.0 Sulphur and its Compounds
5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
Generally, an acid is made by dissolving the corresponding acidic gas (or solid) in water.
Questions 5.3(a)
- State the formula and name of the gas that, when combined directly with water, would form sulphuric (VI) acid, H2SO4.
- Write two equations to represent the two stages through which the gas identified in Question 1 can be prepared. Indicate at least one required condition where necessary.
- Suggest suitable raw materials for the manufacture of sulphuric (VI) acid.
- Manufacturing processes require pure substances. Suggest how the raw materials can be purified before reacting them.
- Some of the reactions are highly exothermic (produce a lot of heat) and require high pressure. Suggest how you would cater for these in the design of sulphuric (VI) acid manufacturing plant.
Answers to Questions 5.3(a) 
NB: Dissolving SO3(g) directly in water is so highly exothermic and explosive and therefore avoided. Instead, SO3(g) is first dissolved in concentrated sulphuric (VI) acid to form oleum (H2S2O7), which is then diluted back to sulphuric (VI) acid.
Figure 5.3 is a flow diagram showing the industrial manufacture of sulphuric (VI) acid by the contact process.
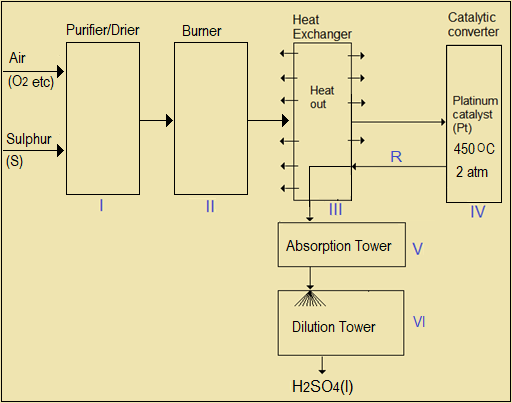
Figure 5.3: Large scale manufacture of sulphuric (VI) acid (the Contact Process)
Questions 5.3(b)
- The drying process in Step I makes use of a liquid. Suggest the identity of the liquid.
- Name the two elements that react with each other in Step II.
- Write an equation for the direct combination of (a) sulphur (VI) oxide with concentrated sulphuric (VI) acid in Step V to form oleum (b) dilution of oleum using water, in Step VI to form concentrated (VI) sulphuric acid.
- Identify two reactive components of the gaseous mixture, R, and explain why the mixture is recycled rather than allowing all of it to flow into the absorption tower.
- Explain why use of elemental sulphur (S), where it is available, is preferred to metal sulphide ores in the sulphuric (VI) acid manufacturing process.
- The catalyst often employed is vanadium (V) oxide (V2O5) rather than platinum (Pt). Give a reason for this (Search in other sources).
- A student argued that "dissolving sulphur (VI) oxide in concentrated sulphuric acid, then diluting the product to give back the acid"
does not make sense, because it assumes that the acid is already manufactured.
- Suggest a possible source of the sulphuric (VI) acid used to dissolve sulphur (VI) oxide.
- Why might this possible source be unsuitable for large scale production of the acid?
- Identify any four factors you would consider before constructing a sulphuric (VI) acid manufacturing plant.
- Like in other industrial plants, it is difficult to completely convert the two oxides of sulphur into sulphuric (VI) acid; yet they are harmful to the environment. Suggest what can be done to the residual gases.
Answers to Questions 5.3(b) 
