CHEMISTRY LEVEL 3
- 1.1 Boyle's Law
- 1.2 Charles'law
- 1.3 Combined gas law
- 1.4 Standard conditions
- 1.5 Diffusion and Graham's law
- 2.1 Relative Mass
- 2.2 Atoms, Molecules and Moles
- 2.3 Compounds and the mole
- 2.4 Empirical and Molecular formula
- 2.5 Concentration of a solution
- 2.6 Molar solutions
- 2.7 Preparation of molar solutions
- 2.8 Dilution of a solution
- 2.9 Stoichiometry of chemical reactions
- 2.10 Volumetric analysis
- 2.11 Titration
- 2.12 Redox titration
- 2.13 Atomicity and molar gas volume
- 2.14 Combining volumes of gases
- 3.1 Alkanes
- 3.1.1 Formulae of alkanes
- 3.1.2 Cracking of alkanes
- 3.1.3 Nomenclature (systematic naming) of alkanes
- 3.1.4 Isomerism in alkanes
- 3.1.5 Laboratory preparation of alkanes
- 3.1.6 Physical properties of alkanes
- 3.1.7 Chemical properties of alkanes
- 3.1.8 Uses of alkanes
- 3.2 Alkenes
- 3.2.1 Nomenclature of alkenes
- 3.2.2 Isomerism in alkenes
- 3.2.3 Laboratory preparation of ethene
- 3.2.4 Physical properties of alkenes
- 3.2.5 Chemical properties of alkenes
- 3.2.6 Test for alkenes
- 3.2.7 Uses of alkenes
- 3.3 Alkynes
- 3.3.1 Nomenclature of alkynes
- 3.3.2 Isomerism in alkynes
- 3.3.3 Laboratory preparation of ethyne
- 3.3.4 Physical properties of alkynes
- 3.3.5 Chemical properties of alkynes
- 3.3.6 Test for alkynes
- 3.3.7 Uses of alkynes
- 3.4 Recommended practice of topic summary
- 4.1 Extraction of nitrogen from air
- 4.2.1 Laboratory preparation of nitrogen gas from the air
- 4.2.2 Laboratory preparation of nitrogen gas from ammonium nitrite (NH4NO2)
- 4.2.3 Uses of nitrogen
- 4.3 Oxides of nitrogen
- 4.3.1 Nitrogen (I) oxide
- 4.3.2 Nitrogen (II) oxide
- 4.3.3 Nitrogen (IV) oxide
- 4.4.1 Laboratory preparation of ammonia
- 4.4.2 Solubility of ammonia in water
- 4.4.3 Reactions of aqueous ammonia (ammonia solution)
- 4.4.4 Reactions of ammonia gas
- 4.4.5 Industrial manufacture of ammonia: The Haber Process
- 4.4.6 Uses of ammonia
- 4.4.7 Nitrogenous fertilizers
- 4.5.1 Laboratory preparation of nitric (V) acid
- 4.5.2 Industrial manufacture of nitric (V) acid
- 4.5.3 Reactions of dilute nitric (V) acid
- 4.5.4 Reactions of concentrated nitric (V) acid
- 4.5.5 Uses of nitric (V) acid
- 4.6.1 Action of heat on nitrates
- 4.6.2 Test for nitrates (nitrate ions, NO3-)
- 4.6.3 Air pollution by nitrogen compounds
- 4.7 Summary on nitrogen and its compounds
- 5.0 Sulphur and its Compounds
- 5.1.1 Extraction of sulphur
- 5.1.2 Allotropes of sulphur
- 5.1.3 Physical properties of sulphur
- 5.1.4 Chemical properties of sulphur
- 5.2.1 Preparation of sulphur (IV) oxide
- 5.2.2 Physical properties of sulphur (IV) oxide
- 5.2.3 Chemical properties of sulphur (IV) oxide
- 5.2.4 Reducing action of sulphur (IV) oxide
- 5.2.5 Oxidization of SO2 to SO3
- 5.2.6 Oxidizing action of sulphur (IV) oxide
- 5.2.7 Test for sulphite (SO32-) and sulphate (SO42-) ions
- 5.2.8 Uses of sulphur (IV) oxide
- 5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
- 5.3.1 Physical properties of concentrated sulphuric (VI) acid
- 5.3.2 Chemical properties of concentrated sulphuric (VI) acid
- 5.3.3 Reactions of dilute sulphuric (VI) acid
- 5.4 Hydrogen sulphide
- 5.4.1 Chemical properties of hydrogen sulphide
- 5.4.2 Air pollution by compounds of sulphur
- 5.5 Summary on sulphur and its compounds
- 6.1 Occurrence of chlorine
- 6.2 Laboratory preparation of chlorine
- 6.3 Physical properties of chlorine
- 6.4 Chemical properties of chlorine
- 6.5 Oxidizing properties of chlorine
- 6.6 Reaction of chlorine with alkaline solutions
- 6.7 Test for chloride ions
- 6.8 Uses of chlorine and its compounds
- 6.9 Preparation of hydrogen chloride gas
- 6.10 Physical properties of hydrogen chloride
- 6.11 Chemical properties of hydrogen chloride
- 6.12 Industrial manufacture of hydrochloric acid
- 6.13 Uses of hydrochloric acid


THE MOLE: Formulae and Chemical Equations: Redox titration
2.0 THE MOLE: Empirical and Molecular formula
2.12 Redox titration
Redox titrations involve both reduction and oxidation reactions at the same time. The procedure is similar to that of neutralization processes (Section 2.11).
Examples are the reactions between acidified potassium manganate (VII) or potassium chromate (VI) as oxidizers, and iron (II), bromide, iodide, sulphite, sulphide, and thiosulphate ions as reducers.
The end-point of a redox titration is sharply marked by a slight excess of one reactant; so it does not need an indicator. In titrating manganate (VII) ions (MnO4-) against sulphite ions (SO32-), for example, end-point is marked by the single extra drop of manganate (VII) ions which suddenly turns the mixture purple or pink (Figure 2.12).
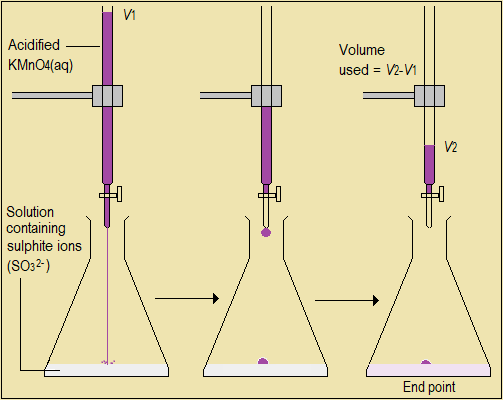
Figure 2.12: Redox titration involving SO32- and MnO4- ions
