CHEMISTRY LEVEL 3
- 1.1 Boyle's Law
- 1.2 Charles'law
- 1.3 Combined gas law
- 1.4 Standard conditions
- 1.5 Diffusion and Graham's law
- 2.1 Relative Mass
- 2.2 Atoms, Molecules and Moles
- 2.3 Compounds and the mole
- 2.4 Empirical and Molecular formula
- 2.5 Concentration of a solution
- 2.6 Molar solutions
- 2.7 Preparation of molar solutions
- 2.8 Dilution of a solution
- 2.9 Stoichiometry of chemical reactions
- 2.10 Volumetric analysis
- 2.11 Titration
- 2.12 Redox titration
- 2.13 Atomicity and molar gas volume
- 2.14 Combining volumes of gases
- 3.1 Alkanes
- 3.1.1 Formulae of alkanes
- 3.1.2 Cracking of alkanes
- 3.1.3 Nomenclature (systematic naming) of alkanes
- 3.1.4 Isomerism in alkanes
- 3.1.5 Laboratory preparation of alkanes
- 3.1.6 Physical properties of alkanes
- 3.1.7 Chemical properties of alkanes
- 3.1.8 Uses of alkanes
- 3.2 Alkenes
- 3.2.1 Nomenclature of alkenes
- 3.2.2 Isomerism in alkenes
- 3.2.3 Laboratory preparation of ethene
- 3.2.4 Physical properties of alkenes
- 3.2.5 Chemical properties of alkenes
- 3.2.6 Test for alkenes
- 3.2.7 Uses of alkenes
- 3.3 Alkynes
- 3.3.1 Nomenclature of alkynes
- 3.3.2 Isomerism in alkynes
- 3.3.3 Laboratory preparation of ethyne
- 3.3.4 Physical properties of alkynes
- 3.3.5 Chemical properties of alkynes
- 3.3.6 Test for alkynes
- 3.3.7 Uses of alkynes
- 3.4 Recommended practice of topic summary
- 4.1 Extraction of nitrogen from air
- 4.2.1 Laboratory preparation of nitrogen gas from the air
- 4.2.2 Laboratory preparation of nitrogen gas from ammonium nitrite ((NH4NO2))
- 4.2.3 Uses of nitrogen
- 4.3 Oxides of nitrogen
- 4.3.1 Nitrogen (I) oxide
- 4.3.2 Nitrogen (II) oxide
- 4.3.3 Nitrogen (IV) oxide
- 4.4.1 Laboratory preparation of ammonia
- 4.4.2 Solubility of ammonia in water
- 4.4.3 Reactions of aqueous ammonia (ammonia solution)
- 4.4.4 Reactions of ammonia gas
- 4.4.5 Industrial manufacture of ammonia: The Haber Process
- 4.4.6 Uses of ammonia
- 4.4.7 Nitrogenous fertilizers
- 4.5.1 Laboratory preparation of nitric (V) acid
- 4.5.2 Industrial manufacture of nitric (V) acid
- 4.5.3 Reactions of dilute nitric (V) acid
- 4.5.4 Reactions of concentrated nitric (V) acid
- 4.5.5 Uses of nitric (V) acid
- 4.6.1 Action of heat on nitrates
- 4.6.2 Test for nitrates (nitrate ions, NO3-)
- 4.6.3 Air pollution by nitrogen compounds
- 4.7 Summary on nitrogen and its compounds
- 5.0 Sulphur and its Compounds
- 5.1.1 Extraction of sulphur
- 5.1.2 Allotropes of sulphur
- 5.1.3 Physical properties of sulphur
- 5.1.4 Chemical properties of sulphur
- 5.2.1 Preparation of sulphur (IV) oxide
- 5.2.2 Physical properties of sulphur (IV) oxide
- 5.2.3 Chemical properties of sulphur (IV) oxide
- 5.2.4 Reducing action of sulphur (IV) oxide
- 5.2.5 Oxidization of SO2 to SO3
- 5.2.6 Oxidizing action of sulphur (IV) oxide
- 5.2.7 Test for sulphite (SO32-) and sulphate (SO42-) ions
- 5.2.8 Uses of sulphur (IV) oxide
- 5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
- 5.3.1 Physical properties of concentrated sulphuric (VI) acid
- 5.3.2 Chemical properties of concentrated sulphuric (VI) acid
- 5.3.3 Reactions of dilute sulphuric (VI) acid
- 5.4 Hydrogen sulphide
- 5.4.1 Chemical properties of hydrogen sulphide
- 5.4.2 Air pollution by compounds of sulphur
- 5.5 Summary on sulphur and its compounds
- 6.1 Occurrence of chlorine
- 6.2 Laboratory preparation of chlorine
- 6.3 Physical properties of chlorine
- 6.4 Chemical properties of chlorine
- 6.5 Oxidizing properties of chlorine
- 6.6 Reaction of chlorine with alkaline solutions
- 6.7 Test for chloride ions
- 6.8 Uses of chlorine and its compounds
- 6.9 Preparation of hydrogen chloride gas
- 6.10 Physical properties of hydrogen chloride
- 6.11 Chemical properties of hydrogen chloride
- 6.12 Industrial manufacture of hydrochloric acid
- 6.13 Uses of hydrochloric acid


Nitrogen and its Compounds: Reactions of ammonia gas
4.0 Nitrogen and its Compounds
4.4.4 Reactions of ammonia gas
Burning of ammonia in oxygen
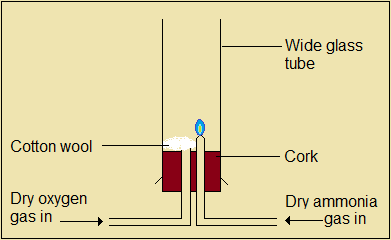
Figure 4.4.4(a): Set-up to burn ammonia in oxygen gas
Ammonia burns in rich oxygen to form two products. One of the products is nitrogen gas.
Questions 4.4.4(a)
- Identify the product that accompanies nitrogen gas in the reaction.
- Describe the product that accompanies nitrogen gas during the reaction.
- Write a chemical equation to represent the burning of ammonia in oxygen.
- State the observations made during the reaction.
- Determine the mass of oxygen gas required to react with 448 cm3 of ammonia, measured at standard states (O =16; molar gas volume at standard states = 22.4 dm3).
- Suggest the function of cotton wool in this set-up.
The following questions refer to the burning of ammonia in oxygen gas.
Answers to Questions 4.4.4(a) 
Direct combination of ammonia with hydrogen chloride gas
Dip one end of a glass rod into concentrated hydrochloric acid then hold it at the mouth of an open bottle containing concentrated ammonia solution (The acid must not drip from the glass rod). Both are fuming liquids producing colourless vapours; so you should use a fume chamber.
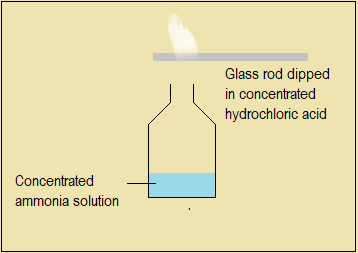
Figure 4.4.4(b): Set-up to demonstrate direct combination between ammonia and hydrogen chloride gases
Questions 4.4.4(b)
- State the observation made on the glass rod.
- Identify the substances that emerge from the bottles containing concentrated hydrochloric acid and concentrated ammonia solution respectively.
- Name the product formed when the two substances combine directly with each other.
- Write an equation to represent the direct combination reaction between the two fumes.
- From this experiment, suggest one method which can be used to identify ammonia gas, other than using red litmus paper.
Referring to Figure 4.4.4(b),
Answers to Questions 4.4.4(b) 
Reduction of copper (II) oxide by ammonia
Ammonia gas acts as a reducing agent, removing oxygen from copper (II) oxide. Like in other reactions, nitrogen is produced as an uncombined gaseous element.
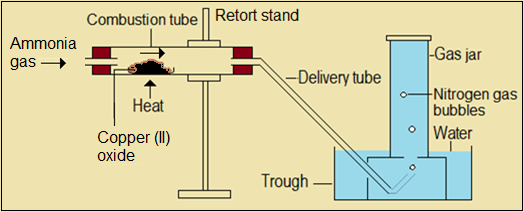
Figure 4.4.4(c): Set-up to react copper (II) with ammonia gas
The following questions relate to Figure 4.4.4(c).
Questions 4.4.4(c)
- State the observation made on (a) copper (II) oxide (b) combustion tube.
- Identify the product that accompanies nitrogen gas in this reaction.
- Write an equation to represent the reaction that takes place in the combustion tube.
- A small amount of ammonia gas may pass over heated copper (II) oxide without reacting with it. Explain why the nitrogen gas bubbles are free from ammonia gas.
- Describe how you would confirm the identity of the product that accompanies nitrogen gas in this experiment.
Answers to Questions 4.4.4(c) 
Catalytic oxidation of ammonia in presence of platinum or copper wire
Heat a copper or platinum wire and hang it as shown in the arrangement. The catalytic oxidation of ammonia produces nitrogen (II) oxide gas.
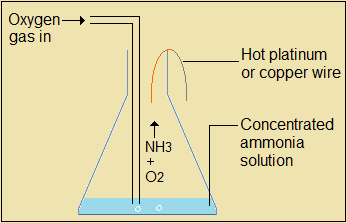
Figure 4.4.4(d): Simple set-up to demonstrate catalytic oxidation of ammonia
The questions that follow are about catalytic oxidation of ammonia.
Questions 4.4.4(d)
- Describe what is observed during catalytic oxidation of ammonia.
- Write the equation for the catalytic oxidation of ammonia.
- What is the evidence that the catalytic oidation is exothermic?
- Eplain the reaction that takes place during the catalytic oxidation of ammonia.
- Suggest how the oxygen gas used in this experiment may be prepared.
Answers to Questions 4.4.4(d) 
