CHEMISTRY LEVEL 3
- 1.1 Boyle's Law
- 1.2 Charles'law
- 1.3 Combined gas law
- 1.4 Standard conditions
- 1.5 Diffusion and Graham's law
- 2.1 Relative Mass
- 2.2 Atoms, Molecules and Moles
- 2.3 Compounds and the mole
- 2.4 Empirical and Molecular formula
- 2.5 Concentration of a solution
- 2.6 Molar solutions
- 2.7 Preparation of molar solutions
- 2.8 Dilution of a solution
- 2.9 Stoichiometry of chemical reactions
- 2.10 Volumetric analysis
- 2.11 Titration
- 2.12 Redox titration
- 2.13 Atomicity and molar gas volume
- 2.14 Combining volumes of gases
- 3.1 Alkanes
- 3.1.1 Formulae of alkanes
- 3.1.2 Cracking of alkanes
- 3.1.3 Nomenclature (systematic naming) of alkanes
- 3.1.4 Isomerism in alkanes
- 3.1.5 Laboratory preparation of alkanes
- 3.1.6 Physical properties of alkanes
- 3.1.7 Chemical properties of alkanes
- 3.1.8 Uses of alkanes
- 3.2 Alkenes
- 3.2.1 Nomenclature of alkenes
- 3.2.2 Isomerism in alkenes
- 3.2.3 Laboratory preparation of ethene
- 3.2.4 Physical properties of alkenes
- 3.2.5 Chemical properties of alkenes
- 3.2.6 Test for alkenes
- 3.2.7 Uses of alkenes
- 3.3 Alkynes
- 3.3.1 Nomenclature of alkynes
- 3.3.2 Isomerism in alkynes
- 3.3.3 Laboratory preparation of ethyne
- 3.3.4 Physical properties of alkynes
- 3.3.5 Chemical properties of alkynes
- 3.3.6 Test for alkynes
- 3.3.7 Uses of alkynes
- 3.4 Recommended practice of topic summary
- 4.1 Extraction of nitrogen from air
- 4.2.1 Laboratory preparation of nitrogen gas from the air
- 4.2.2 Laboratory preparation of nitrogen gas from ammonium nitrite (NH4NO2)
- 4.2.3 Uses of nitrogen
- 4.3 Oxides of nitrogen
- 4.3.1 Nitrogen (I) oxide
- 4.3.2 Nitrogen (II) oxide
- 4.3.3 Nitrogen (IV) oxide
- 4.4.1 Laboratory preparation of ammonia
- 4.4.2 Solubility of ammonia in water
- 4.4.3 Reactions of aqueous ammonia (ammonia solution)
- 4.4.4 Reactions of ammonia gas
- 4.4.5 Industrial manufacture of ammonia: The Haber Process
- 4.4.6 Uses of ammonia
- 4.4.7 Nitrogenous fertilizers
- 4.5.1 Laboratory preparation of nitric (V) acid
- 4.5.2 Industrial manufacture of nitric (V) acid
- 4.5.3 Reactions of dilute nitric (V) acid
- 4.5.4 Reactions of concentrated nitric (V) acid
- 4.5.5 Uses of nitric (V) acid
- 4.6.1 Action of heat on nitrates
- 4.6.2 Test for nitrates (nitrate ions, NO3-)
- 4.6.3 Air pollution by nitrogen compounds
- 4.7 Summary on nitrogen and its compounds
- 5.0 Sulphur and its Compounds
- 5.1.1 Extraction of sulphur
- 5.1.2 Allotropes of sulphur
- 5.1.3 Physical properties of sulphur
- 5.1.4 Chemical properties of sulphur
- 5.2.1 Preparation of sulphur (IV) oxide
- 5.2.2 Physical properties of sulphur (IV) oxide
- 5.2.3 Chemical properties of sulphur (IV) oxide
- 5.2.4 Reducing action of sulphur (IV) oxide
- 5.2.5 Oxidization of SO2 to SO3
- 5.2.6 Oxidizing action of sulphur (IV) oxide
- 5.2.7 Test for sulphite (SO32-) and sulphate (SO42-) ions
- 5.2.8 Uses of sulphur (IV) oxide
- 5.3 Large scale (industrial) manufacture of sulphuric (VI) acid
- 5.3.1 Physical properties of concentrated sulphuric (VI) acid
- 5.3.2 Chemical properties of concentrated sulphuric (VI) acid
- 5.3.3 Reactions of dilute sulphuric (VI) acid
- 5.4 Hydrogen sulphide
- 5.4.1 Chemical properties of hydrogen sulphide
- 5.4.2 Air pollution by compounds of sulphur
- 5.5 Summary on sulphur and its compounds
- 6.1 Occurrence of chlorine
- 6.2 Laboratory preparation of chlorine
- 6.3 Physical properties of chlorine
- 6.4 Chemical properties of chlorine
- 6.5 Oxidizing properties of chlorine
- 6.6 Reaction of chlorine with alkaline solutions
- 6.7 Test for chloride ions
- 6.8 Uses of chlorine and its compounds
- 6.9 Preparation of hydrogen chloride gas
- 6.10 Physical properties of hydrogen chloride
- 6.11 Chemical properties of hydrogen chloride
- 6.12 Industrial manufacture of hydrochloric acid
- 6.13 Uses of hydrochloric acid


Organic Chemistry 1: Recommended practice of topic summary
3.0 Organic Chemistry 1
3.4 Recommended practice of topic summary
For better retention of what is learnt, the learner is encouraged to prepare, for every topic read, a condensed one-page summary notes and a reaction scheme (flow chart, or map). Begin with a simple scheme, then build and enrich it gradually as you revise and understand the topic more. Refer to "Answers to Questions" sections, where you find it necessary, to enable you write the summary. Here is an example for alkenes.
Alkenes
Unsaturated hydrocarbons with carbon-carbon double bonds
Laboratory preparation of ethene (an alkene)
Dehydration of ethanol using concentrated sulphuric (VI) acid. CH3CH2OH H2SO4(l) H2O + CH2CH2
Industrial production
From cracking of higher alkanes
General formula
Belong to a homologous series with the general formula, CnH2n
Nomenclature of alkenes
General name: Alk-x-ene where x is the position of the double bond.
Example: CH3CH2CH2CHCHCH3 is Hex-2-ene
Physical properties of alkenes:
Largely insoluble in water; soluble in organic (oily) solvents e.g. tetrachloromethane, colourless, low density, low boiling and melting points.
Chemical properties of alkenes
Ethene extinguishes a glowing splint, is neutral, decolourizes chlorine water, bromine water, potassium manganate (VII) solution, and potassium chromate (VI) even in the dark.
With halogens (Cl2, Br2, I2, F2), e.g. C2H2(g) + Cl2(g) CHClCHCl(g) (halogenation/chlorination, etc)
With hydrogen e.g. CH2CH2(g) + H2(g) Ni/140 OC CH3CH3(g) (hydrogenation),
With hydrogen halides, e.g. CH2CH2 + HCl(g) CH3CH2Cl (hydro-halogenetation)
With chlorine water, CH2CH2(g) + HOCl(aq) CH2ClCH2OH(l)
Self-addition reaction: nCH2=CH2 (-CH2CH2-)n (addition polymerization)
Test for alkenes
Alkenes decolourize chlorine water, bromine water, and potassium manganate (VII) even in the dark.
Uses of alkenes
Welding fuel (ethyne), manufacture of polythene and plastics, and alcohol
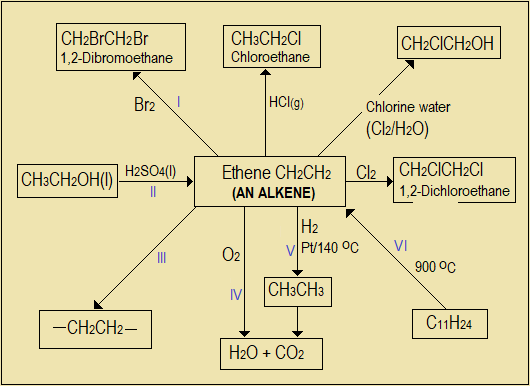
Figure 3.4: Reaction scheme (map) for ethane
NB: The reaction scheme does not show all the products and equations for each step. The learner should practice writing them on a separate workbook.
Questions 3.4(a)
- Name the specific addition reactions and processes labelled I, II, III, IV, V, VI in Figure 3.4.
- Prepare a condensed summary together with a reaction scheme for alkanes, using ethane as an example.
Answers to Questions 3.4(a) 
Questions 3.4(b)
- From what we have learnt in this topic, explain how you would test for each of the following groups of substances.
- Alkanes (e.g. ethane)
- Alkenes (e.g. ethene)
- Alkynes (e.g. ethyne)
- Tests for the substances named in Question 1 assume that they have already been confirmed as hydrocarbons. That is, their products of combustion in oxygen are carbon (IV) oxide (CO2) and water (H2O) only. From your prior knowledge of chemical behavior of carbonates, acids, hydrated salts, oxygen and water, describe how each of the following substances can be confirmed.
- Carbon (IV) oxide (CO2)
- Water (H2O)
- Hydrogen gas (H2)
- Oxygen gas (O2)
Answers to Questions 3.4(b) 
3.5 Project 3
Design, preferably in a group, an experiment to determine factors that make the burning of oil fuels more efficient, to reduce energy losses. In the design, explain clearly the procedure you would follow, the materials and apparatus you require and how you would use them, the measurements you would take, and how you would use them to identify the factors.
